
The overuse of antibiotics has led to antimicrobial resistance, now with scientific society looking out for potential alternatives not only for human usage but also for the food and farming industry, poultry, dairy, and aquaculture. The therapeutic failures have an economic burden to society, and to counter these effects, many new novel approaches have been under research, of which using bacteriophages is promising.
Phage therapy study in veterinary medicine is on the rise, especially for livestock, poultry, and aquaculture species, which are being explored. Recently, researchers from the Department of Veterinary Medicine and Animal Sciences—DIVAS, Università degli Studi di Milano, Italy, have suggested reducing or even avoiding antibiotics in aquaculture and using phages instead.
According to the FAO (Food and Agricultural organization of the United Nations) report, Global consumption of aquatic foods has risen. The report states, “Of total aquatic animal production, 89 percent was used for direct human consumption, underscoring the critical role of fisheries and aquaculture in maintaining global food security”. However, bacterial diseases remain the major challenge in aquaculture, resulting in huge economic losses. Aquatic animals are susceptible to numerous bacterial infections or co-infections, as they are exposed to various pathogenic microorganisms in their environment.
Phage Utilization in Aquaculture
“In the absence of effective vaccines that could prevent the majority of diseases affecting aquatic species, or due to the practical impossibility of vaccinating fish at early life stages because of their small size or immature immune system, phage therapy plays the dual role of therapeutic and preventive measures against bacterial infections in aquaculture. This is especially important during larval production, before the fish are introduced into aquaculture containers. Furthermore, phages can effectively lyse bacteria in both planktonic and biofilm states,” says the study.
Researchers say, “Phage therapy can be approached in two ways: ‘sur mesure’ (tailored for individual cases) and ‘prêt-à-porter’ (a universal, one-size-fits-all method).” Phages are collected from natural and wastewater sources, isolated and characterized in the laboratory, and engineered before being tested in vitro. The selected modified phages are then tested in vivo on infected animals.
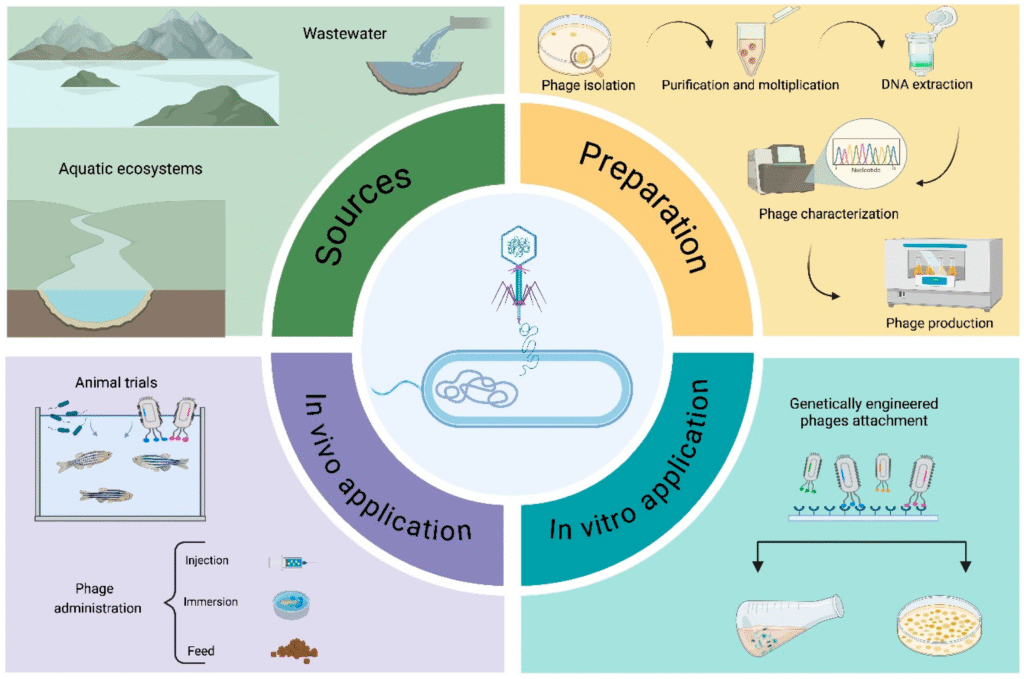
The studies so far
Bacterial species belonging to the genera Aeromonas, Vibrio, Edwardsiella, Streptococcus, and Flavobacterium, are particularly pathogenic for fish, mollusks, and crustaceans. Many in vivo studies have been conducted in larval models in Zebra Fish. For example, Aeromonas species are pathogenic for freshwater fish and other animal species. It is a zoonotic pathogen and is also involved in biofilm formation. A study has suggested using a single phage against Aeromonas species, others have demonstrated that using a cocktail of phages is more effective for resolving infections. All the routes were effective against Aeromonas infection; however, intraperitoneal injection proved to be the most effective for both systemic and localized infections
Vibrio spp. are present in marine, estuarine, and freshwater environments and are responsible for vibriosis, the most common disease of marine and estuarine fish, both in natural and farm systems worldwide. However, Vibrio species infections can also affect freshwater fish. The CHOED bacteriophage was selected, characterized, and tested in vivo through immersion in salmon. This phage demonstrated up to 100% survival in experimental infection with pathogenic V. anguillarum.
Edwardsiella species infects commercially important fish species, including turbot, channel catfish, tilapia, and flounder. It enters through the gut and causes edwardsiellosis and enteric septicemia, leading to high mortality rates among economically important cultured fish species and considerable economic losses in the global aquaculture industry. A lytic phage, ETP-1 (Podoviridae), first isolated from a marine fish farm, was characterized for its effectiveness against Edwardsiella.
Streptococcus species causes streptococcosis, a catastrophic disease in freshwater and saltwater aquaculture. Symptoms include septicemia, meningoencephalitis, irregular swimming, loss of buoyancy control, and spinal abnormalities. Vaccination is an effective and safe strategy against Streptococcus infections; however, in the absence of vaccines and potentially effective bacteriophages, phage-derived or synthetic endolysins can be experimented with.
Flavobacterium columnare is responsible for epidermal infections in farmed fish worldwide, leading to high mortality rates. The disease is primarily epidermal and transmitted through water, affecting the skin, fins, and gills of fish. A single administration of the phage in small-scale experimental systems (inflow-through tanks) was sufficient to rescue the rainbow trout population. Phage FCL-2, administered by immersion, also significantly increased survival in Atlantic salmon after experimentally challenging the yolk sac fry with F. columnare. Additionally, the phage treatment reduced the pathogen in the water without affecting the bacterial community composition of the rearing water.
Citrobacter species is responsible for infections in aquaculture species, leading to co-morbidity between humans and fish. Finfish, crayfish, catfish, stingrays, angelfish, tilapia, carp, doctor fish, and eels can all be infected with Citrobacter. Clinical manifestations include reduced mobility, gill erythema or lesions in the tail or fin areas. One study identified two phages, Citrophage MRM19 and Citrophage MRM57 (Sydoviridae and Podoviridae, respectively), and tested both in vitro and in vivo in a zebrafish model.
Pseudomonas aeruginosa, which is β-lactamase-resistant, has been isolated from catfish species, where they cause ulcerative lesions. Lytic bacteriophages targeting P. aeruginosa have been isolated, characterized in vitro, and tested in vivo (topically administered at 1010 PFU/mL to the skin), showing effectiveness in treating the ulcerative lesions in infected catfish.
More studies are needed
The isolation, identification, whole genome sequencing, and genomic analysis of bacteriophages are essential for obtaining detailed information about the phages and establishing phage libraries. These libraries are necessary for preparing effective phage cocktails specifically tailored to pathogens identified by local farmers in a target region. The major challenge in aquaculture is ensuring the efficient delivery of phages to the infection site and addressing potential immune-mediated phage clearance.
Phage therapy is crucial for reducing mortality due to bacterial infections in aquaculture. A successful application requires careful planning of the timing, dose, and frequency of phage delivery, taking into account the bacterial pathogen’s virulence characteristics, the nature of the outbreak. Several factors influence the efficacy of phage therapy in aquaculture, including the fish’s weight, the required phage dose, early disease diagnosis, and environmental conditions. These conditions encompass the physical and chemical characteristics of the water, such as salinity, organic matter content, temperature, and pH. Phage therapy needs more understanding of the susceptibility of virulent bacterial strains, their genetic traits involved in pathogenicity, and the phage–bacterium co-evolution.
Phage therapy in aquaculture is considered an effective alternative to antibiotics for treating bacterial diseases in the “One-Health” approach, and is still in the early stages of development, but it shows much potential.
Read the full article herehttps://www.mdpi.com/2076-2607/13/4/831
Leave a Reply
You must be logged in to post a comment.