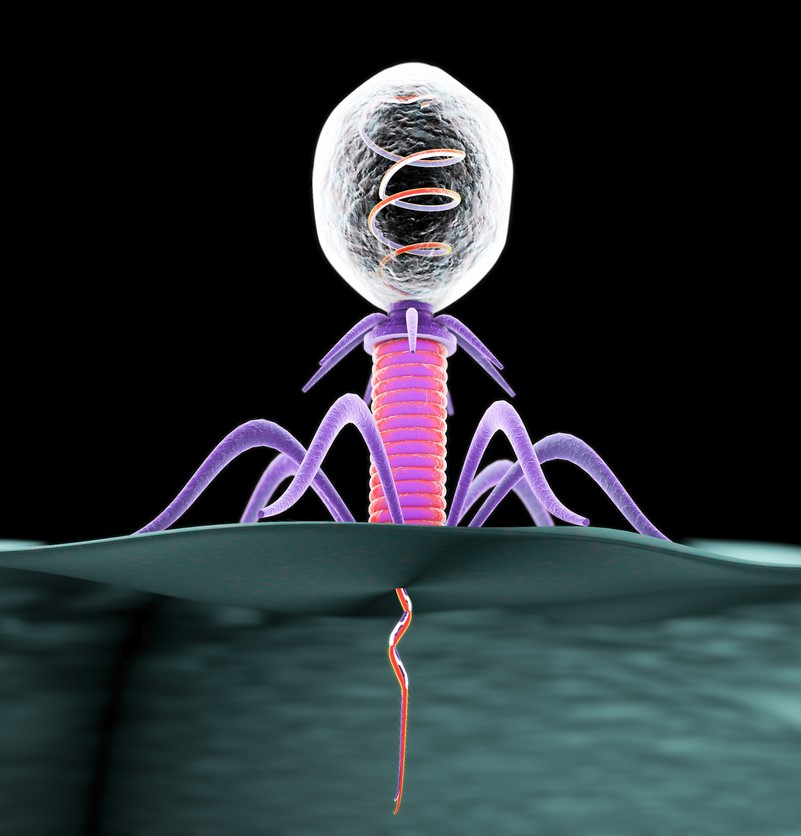
In 2017, the World Health Organization (WHO) highlighted the particular threat of Gram-negative pathogens resistant to multiple antibiotics. The Discovery, design, and development of new and alternative antibacterial therapies are crucial. This review concerns the therapeutic use of bacteriophage (phage): viruses that exclusively infect bacteria and can act as bactericidal agents. This approach of “phage therapy” is an old idea that is recently regaining popularity. Efforts are buoyed by the development of easier methods for engineering phage for different purposes in biotechnology. Also, the extreme biodiversity of phages in nature can be leveraged for “bioprospecting”: the discovery and development of naturally evolved phages with properties that are ideal for phage therapy use (e.g., Chan et al., 2018). Below we examine the past, present, and future uses of phage therapy, especially addressing how this newly energized field may proceed with modern, rational therapeutic approaches.
Phage Therapy: A Renewed Approach
It has become much easier to define and test phages as antibacterial treatments. Today’s technology allows for low-cost whole-genome sequencing, automated microbe growth monitoring, and effective high-throughput approaches for screening hundreds or even thousands of samples at once. Modern clinical trials should be carefully planned to be safer, more inclusive, and (if possible) produce useful data compared to prior attempts, it is becoming increasingly understood. Phage therapy trials should be double-blinded, placebo-controlled, and comprise large, diverse cohorts. They may also be planned to collect pertinent longitudinal data from clinical isolates. To investigate a variety of basic and clinical microbiology assays, researchers could, for instance, undertake follow-up lab studies and whole-genome sequencing of phage and/or bacteria obtained during treatment. Also, our increased understanding of the human microbiome and its interactions with human immunology warrant closer investigation of possible phage and immune system interactions in clearing infections.
However, one obvious limitation to phage therapy is the inevitable evolution of phage resistance in bacteria. Modern approaches to phage therapy should both acknowledge and capitalize on this certainty. Evolutionary biology describes how genetic trade-offs should be widely observed in biological systems; organisms sometimes evolve one trait that improves fitness (a relative advantage in survival or reproduction) while simultaneously suffering reduced performance in another trait. Phage therapy would thus benefit from utilizing certain phages which select the target bacterial pathogen to suffer specific genetic trade-offs. In particular, if the proximate binding of a lytic phage is known to associate with a virulence factor or mechanism for antibiotic resistance in the target bacteria, this should exert strong selection for the bacteria to mutate or downregulate the phage-binding target(s). This approach should be especially useful in the case of opportunistic bacterial pathogens because the bacteria could evolve reduced virulence or antibiotic resistance and still thrive in a different ecological setting (e.g., soil) as opposed to “arms-race” selection for escalating virulence in an obligate pathogen such as in response to vaccine pressure (e.g., Marek’s disease virus in chickens; Nair, 2005). Thus, this approach to phage therapy should be doubly effective; success is achieved when phage lyses the target bacterium, but also when bacteria evolve phage resistance because they suffer reduced virulence or increased sensitivity to antibiotics. In the following sections, we return to this paradigm of phage-imposed genetic trade-offs.
A phage that requires a virulence factor to attach to and infect a bacterium may select against the expression of that virulence factor. Selection against virulence factors could be multiply effective, as some virulence factors such as capsules have been shown to hide antigenic sites, provide some degree of antibiotic resistance, and prevent phagocytosis by macrophages. Phages that use components of LPS as receptors select against the expression of these components typically resulting in “rough” colony-forming mutants through phase variable expression of LPS, point mutations, or even large chromosomal deletions in LPS biosynthesis genes. While resistant to LPS targeting phage, these bacterial mutants are typically reduced in both fitness and virulence. Selection against other virulence factors that can serve as phage receptors such as adhesins, pili, or secretion systems could prevent bacterial attachment and invasion of epithelial cells.
Certain lytic phages may be more effective in phage therapy because they kill target bacteria while simultaneously imposing strong selection against bacterial virulence or antibiotic resistance when bacteria mutate to avoid phage attack. Phage that uses antibiotic efflux pumps as receptors (red) can select for phage-resistant bacterial mutants with impaired efflux pumps; these phage-resistant bacterial mutants are more sensitive to antibiotics. Phages that bind to structural virulence factors such as a capsular antigen (purple) can select for phage-resistant bacterial mutants that lack the capsule; these non-capsulated phage-resistant mutants are less virulent because they are more easily engulfed by phagocytic cells.
Similarly, phages that attach to an antibiotic efflux pump to infect may select against the expression of the efflux pump, rendering the bacteria more sensitive to antibiotics that were previously effluxed. For example, phage TLS selected for tolC and rfa mutants in E. coli at a typical frequency of 10−5 to 10−6. The TLS-resistant mutants with altered TolC were hyper-sensitive to novobiocin. Additionally, when phage-resistant mutants were selected in the presence of novobiocin, the frequency of recovered mutants decreased 1,000-fold. More recently, it was demonstrated that phage OMKO1 associates with the outer membrane protein M (OprM) of MexAB- and Mex-XY-OprM efflux pumps of the opportunistic pathogen P. aeruginosa. This interaction selects for phage-resistant mutants that are sensitive to antibiotics, as a “genetic trade-off.” Chan et al. demonstrated that phage-resistant mutants, in both lab strains and clinical isolates of P. aeruginosa, were more sensitive to antibiotics, including ceftazidime. This was likely due to mutations or deletions in the operon encoding for the multidrug efflux pump resulting in nonfunctional gene products. Hypothetically, this promising result might also occur in other bacterial pathogens with similar modes of achieving broad antibiotic resistance via homologous or convergent efflux pump mechanisms. Overall, thoughtful consideration of the inevitable evolution of phage resistance during treatment could greatly benefit phage therapy efforts.
Animal Models for Efficacy
Animal studies can help bridge the gap between in vitro studies and the actual clinical application of phage therapy. Unfortunately, most animal models investigate acute infections, which may not be the ideal analog for phage therapy targeting chronic infections in humans. Many of these studies observe best results when phages are applied simultaneously with the bacterial challenge, which will not necessarily be applicable in the clinic. In many cases, no measures were taken to check for the in vivo evolution of phage resistance by bacteria. Also, the comparison of phage treatment to antibiotic treatment or even a combination of phage and antibiotic treatments is only beginning to be investigated in animal models. Nevertheless, animal models provide vitally useful data on the efficacy and safety of phage therapy in living hosts and are crucial for the further development of the approach.
Systemic Infections
Several studies have investigated the efficacy of phage therapy in the treatment of systemic infections. In a gut-derived model of P. aeruginosa sepsis, Watanabe et al. (2007) observed 67% survival of infected mice when phage therapy was administered orally 1 day post-infection. Capparelli et al. (2007) observed that successful protection of mice with a systemic Staphylococcus aureus infection depended on phage dose; Biswas et al. (2002) observed similar results of dose-dependent success in a mouse model of vancomycin-resistant Enterococcus faecium bacteremia. In a systemic disease model of Vibrio vulnificus, successful control of disease was only achieved when bacterial infection and phage treatment were administered simultaneously. The determinants of success for phage therapy to treat systemic infections are likely dependent on multiple factors which need to be thoroughly examined prior to the widespread use of phage as a treatment for sepsis in humans.
Local Infections
Phage therapy for localized infections (e.g., otitis, urinary tract infections, infected burns) is recognized for its potential to entirely circumvent the use of chemical antibiotics. Furthermore, the use of chemical antibiotics for surgical and hospital-acquired infections is limited, as these often constitute the strains with the greatest antibiotic resistance. Watanabe et al. (2007) observed 92% survival of mice with an intraperitoneal P. aeruginosa infection treated simultaneously with phage. A similar study of S. aureus abscesses in mice by Capparelli et al. (2007) enumerated the reduction in bacterial load resulting from phage therapy and observed that phage applied concurrently with bacteria prevented the formation of abscesses. When administered 4 days after bacterial challenge, a single dose of phage resulted in a 100-fold reduction in bacterial load, whereas multiple doses of phage resulted in a 10,000-fold reduction. In a mouse model of P. aeruginosa infection of burn wounds, phage treatment improved the survival rate from 6% in the untreated controls to 88% when phages were administered via intraperitoneal injection 72 h post-infection. In contrast, phage treatment only resulted in 22% or 28% survival when administered subcutaneously or intramuscularly. Further pharmacokinetic studies demonstrated that phage delivered intraperitoneally persisted at higher levels in the liver, spleen, and blood than phage delivered intramuscularly or subcutaneously. Finally, a murine model was used to investigate the ability of phage to treat an E. coli urinary tract infection. Phage administered intraperitoneally 24 h after bacterial challenge resulted in a 100-fold reduction in bacterial load in the kidneys 48 h after phage treatment. The same phage resulted in a significant reduction in bacterial load in an E. coli pneumonia model but was ineffective in an E. coli model of sepsis.
Gastrointestinal Infections
Applying phage therapy to gastrointestinal bacterial infections could potentially reduce or prevent the colonization of virulent bacteria without disrupting the natural gut flora. Galtier et al. (2017) observed that a preventative treatment of phage, 4 days after an adherent-invasive E. coli challenge, was able to reduce bacterial colonization in the gut of dextran sodium sulfate-treated mice and prevented the progression of colitis symptoms. In an insect model of Clostridium difficile colonization, prophylactic treatment with phage 2 h prior to bacterial challenge resulted in 100% survival, while simultaneous administration of phage and bacteria resulted in 72% survival, and phage administration 2 h post bacterial challenge resulted in 30% survival. Yen et al. (2017) observed that prophylactic treatment with a phage cocktail was able to reduce V. cholerae colonization in the small intestine of infant mice when phages were provided 3 and 6 h prior to bacterial challenge. However, phage-resistant bacterial mutants were recovered after treatment, and effects of phage treatment were reduced when administered more than 6 h before bacterial challenge and when mice were challenged with a higher dose of V. cholerae. While the result of prophylactic treatment of gastrointestinal infections with phage is generally favorable, more studies that provide treatment after bacterial challenges, such as Galtier et al. (2017), are needed, as prophylactic treatment is not always possible in the clinic.
Lung Infections
Phage therapy for the treatment of lung infections, particularly chronic lung infections which are common in those with cystic fibrosis (CF), has seen renewed interest recently with the increase in MDR bacteria associated with the lung. Waters et al. (2017) observed complete eradication of a CF strain of P. aeruginosa in mice when two doses of phage were administered intranasally to infected mice 24/36 or 48/60 h after infection. Treatment at 144/156 h post-infection resulted in complete eradication of infection in 70% of mice and a significant reduction in the remaining 30%. In another CF lung infection model, phage treatment significantly improved the survival rate of mice when administered intranasally at 2 h post-infection. Interestingly, a high dose of phage administered 4 days prior to the bacterial challenge provided complete protection to mice, indicating that prophylactic treatment with phage could prevent chronic infections. Semler et al. (2014) investigated different routes of administration of phage in a mouse model of Burkholderia cepacia complex respiratory infection. A 100-fold decrease in bacterial load was observed when phage was administered via nebulization, while no decrease was observed when administered via intraperitoneal injection. Promising results for both prophylactic and curative treatment of lung infections with phage indicate that these types of infections may be a reliable target for effective phage therapy.
Antibiotic and Phage in Combination
While there have been many in vivo studies on the efficacy of phage therapy, not many recent studies have compared the in vivo efficacy of phage therapy to that of antibiotics or even combined phage and antibiotic treatment. Huff et al. (2004) investigated the efficacy of traditional antibiotics, phage treatment, or a combination of both in a head-to-head trial in an E. coli challenge in broiler chickens. The standard of care treatment, enrofloxacin (fluoroquinolone), reduced mortality from 68% in untreated birds to 3%, while phage treatment alone reduced mortality to 15%. Combination therapy of phage and enrofloxacin resulted in no mortality. Similarly, Oechslin et al. (2017) observed that phage in combination with ciprofloxacin resulted in a 10,000-fold greater reduction in bacterial load as compared to phage or ciprofloxacin treatment alone in rats with experimental endocarditis due to P. aeruginosa. Furthermore, they noted that this particular combination of phage and antibiotics resulted in the synergistic killing of P. aeruginosa both in vitro and in vivo. As the future of phage therapy will likely be that of combined therapy with chemical antibiotics, additional studies examining potential synergy between phage and antibiotics both in vitro and in vivo are needed.
Compared to phage therapy studies in vivo animal models, there have been relatively few reports on the clinical use of phage and even fewer controlled clinical trials.
Phage therapy, long overshadowed by chemical antibiotics, is garnering renewed interest in Western medicine. This stems from the rise in frequency of multi-drug-resistant bacterial infections in humans. There also have been recent case reports of phage therapy demonstrating clinical utility in resolving these otherwise intractable infections. Nevertheless, bacteria can readily evolve phage resistance too, making it crucial for modern phage therapy to develop strategies to capitalize on this inevitability. Here, we review the history of phage therapy research. We compare and contrast phage therapy and chemical antibiotics, highlighting their potential synergies when used in combination. We also examine the use of animal models, case studies, and results from clinical trials. Throughout, we explore how the modern scientific community works to improve the reliability and success of phage therapy in the clinic and discuss how to properly evaluate the potential for phage therapy to combat antibiotic-resistant bacteria.