In a groundbreaking study, researchers at ETH Zurich have uncovered a potential game-changer in the ongoing battle against antibiotic-resistant bacteria. Contrary to conventional wisdom, the team has discovered that certain bacteriophages, or viruses that infect bacteria, can successfully target dormant bacteria, providing a promising alternative to traditional antibiotics.
Dormant bacteria are often referred to as persister cells. Persister cells are a subpopulation of bacterial cells that have the ability to enter a dormant or non-growing state, making them less susceptible to antibiotics. These cells can survive antibiotic treatment, contributing to the persistence of bacterial infections and posing challenges in therapeutic interventions.
Most bacteria, when faced with nutrient deficiency or stress, enter a controlled resting state, shutting down their metabolism to survive. This dormant state shields them from conventional antibiotics and bacteriophages. Traditionally, bacteriophages primarily target actively growing bacterial cells. This notion was based on the idea that phages require specific processes occurring in actively metabolizing cells for successful infection and replication. Contrary to findings in various other studies, this recently published research demonstrates that the situation is different.
The journey to this groundbreaking discovery began in 2018 when the research team initiated their project. Expecting to isolate around 20 phages within the first year, it wasn’t until 2019 that a lucky breakthrough occurred in the form of a previously unknown virus found in rotting plant material near Riehen. This virus proved to be the first documented phage capable of attacking bacteria in a dormant state.
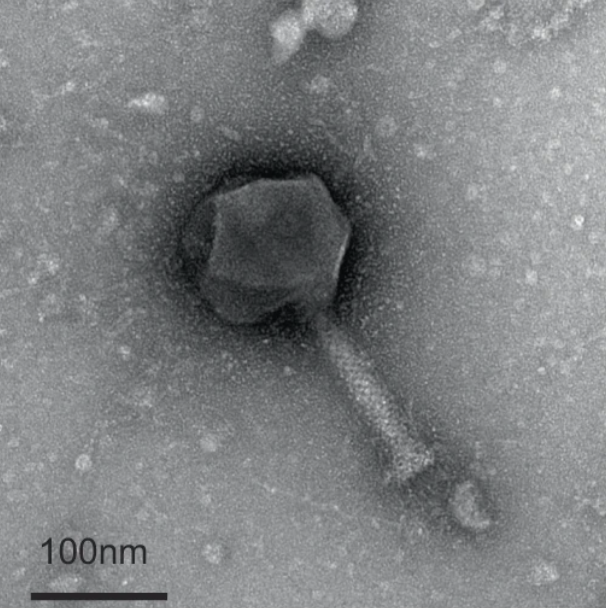
Paride, as the researchers named their newfound phage, demonstrated its effectiveness against Pseudomonas aeruginosa, a widespread bacterium responsible for various infections, including serious respiratory diseases like pneumonia. While the exact mechanism by which Paride awakens dormant bacteria is not yet clear, the researchers suspect it employs a specific molecular key to initiate the awakening process.
To assess Paride’s efficacy, the researchers conducted tests in conjunction with the antibiotic meropenem. The combination of Paride phages and the antibiotic proved highly effective in eradicating dormant bacteria, showing promise not only in laboratory settings but also in experiments involving mice with chronic infections.
While challenges remain, the groundbreaking discovery of phages targeting dormant bacteria opens new avenues for combating persistent bacterial infections and underscores the potential of viral strategies in the ongoing battle against antibiotic resistance. As research progresses, these findings could pave the way for innovative treatments and reshape the future of infectious disease management.
Read about their published paper here Maffei, E., Woischnig, AK., Burkolter, M.R. et al. Phage Paride can kill dormant, antibiotic-tolerant cells of Pseudomonas aeruginosa by direct lytic replication. Nat Commun 15, 175 (2024). https://doi.org/10.1038/s41467-023-44157-3



Comments