 |
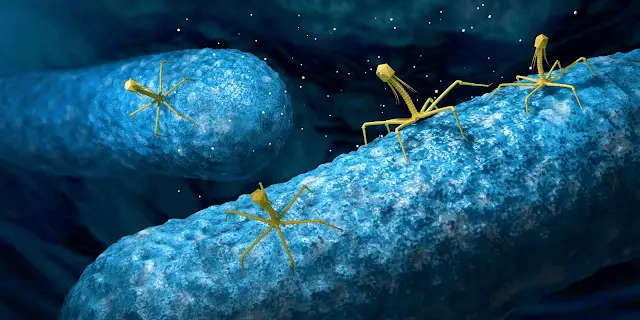
| Structure of gram-negative bacteriophage lysin. credit Fischetti (2019) |
Phage lysins are specialized hydrolytic cell wall enzymes that are used to rupture phage-infected bacteria and release progeny phage particles into the environment. Phage lysins have also been studied and used on their own as a potential therapy to treat bacterial infections. They are potent, fast-acting, specific to a small number of bacteria, and relatively harmless to other cell types. Phage lysins can also kill bacteria that are dormant (Fischetti, 2010) and have been used to treat short-term infections in mice (Gilmer et al., 2013). Leverentz and colleagues have used a number of phage lysins to kill various Gram-positive pathogens while (Nelson et al., 2001) reported the prophylactic use of phage lysins in an in vivo model. Loeffler et al., 2001 also investigated the use of phage lysins to control S. pneumoniae. Phage therapy for bacterial infection has been around for over 50 years (Bertoye et al., 1959; Soohill, 2013), but recent developments using lysins and live phages are very promising.
Data show that phage lysins in combination with antibiotics improve clinical outcomes in patients with bacteremia, including endocarditis, compared to antibiotics alone. Individualized phage therapy was successfully used by Robert T. Schooley and others to treat a case of multidrug-resistant Acinetobacter baumannii in the United States in 2015. Yale University under the Yale Charitable Use Program. Phage therapy primarily uses obligate lytic phages to kill their respective host bacteria, leaving human cells intact and reducing the wider exposure to commensal bacteria that often results from the use of antibiotics.
Phage therapy is the therapeutic use of phages to treat pathogenic bacterial infections. Currently, bacteriophages are used to treat bacterial infections that cannot be treated with conventional antibiotics, especially in Russia and Georgia. The ability to combine antibiotic therapy and phage therapy, the use of phage cocktails, and previously unexplored phage protein products are the most promising areas for the effective treatment of drug-resistant bacterial infections.
Phage therapy is an exciting area of rediscovered research with many applications in science, agriculture, veterinary medicine, and medicine, including potential antibiotic-resistant pathogen prevention. Numerous studies have highlighted the potential of therapeutic phages in vitro and in vivo, and although many clinical trials have been conducted over the past decade, more data are needed to provide a strong regulatory rationale for clinical use. Although the Phage community scientists' studies represent a breakthrough in medical phage research, in vitro and clinical studies are still needed to gain widespread acceptance of the use of therapeutic phages to treat people with pathogenic diseases or MDR.
The same is true for bacteriophages, which are used in many animal models of infection to study phages as potential treatments, especially in the context of antibiotic-resistant infections. In the most recent case of using phages for compassionate care, the medical team took a holistic approach, arguing that one or even two phages might at some point become ineffective due to the emergence of bacterial resistance. For example, in last year's Isabelle Carnell-Holdway case, a 15-year-old cystic fibrosis patient was being treated for a disseminated M. abscessus infection using three bacteriophages (Phage cocktail).
More recently, phage therapy approaches have been applied to systemic infections (either direct or using special matrix) and even intracellular infections, as well as to the addition of non-replicating and isolated phage enzymes such as lysins to the antimicrobial arsenal. Phage therapy is most effective in ulcers with bacterial agents, however, a personalized phage therapy strategy could also eliminate pathogens in co-infections. Phage therapy is now considered a good way to suppress antibiotic-resistant bacteria. Only a few countries on the planet are officially using bacteriophages for bacterial therapy, and as technology improves, other regions are experimenting with phage therapy. Phages have shown great potential in the treatment of antibiotic-resistant bacterial infections.
Studies describe the use of phage lysins to identify promising lead molecules with reduced antimicrobial development potential. While antibiotics often kill bacteria indiscriminately, lysins (just like phages) are highly specific and do not interfere with normal symbiotic microflora.
Lysins have also been shown to have the potential to control and eliminate phytopathogens. In particular, the use of phage-encoded lysins or cell wall hydrolases is of particular interest because of its potent and often species-specific lysine activity and the apparent lack of bacterial resistance to lysine activity (Read about phage resistance). During the phage life cycle of bacterial hosts, lysins are expressed during viral replication and are ultimately used to lyse peptidoglycan, lyse bacteria and release progeny virions.
The existence of lysine-resistant bacteria is not possible because phages have naturally evolved with their bacterial hosts over millions of years to produce these enzymes needed to release phage progeny. At the same time, host bacteria can use their defense systems to prevent phage reproduction, making it difficult to find suitable phages that can overcome bacterial limitations.
Phage therapy using the whole virus brings a lot of confusion among communities and creates fear despite being harmless. Also, approval processes have been regarding phage as a whole need to be more purified to remain with only active compounds. The use of Lysin is the right answer for that In fact, some biotech companies have already gone the extra mile to prepare counter-ready lysin-based products for Skincare and some other products paving a way for more products.
Cited Sources
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3035150/ 0
https://www.dovepress.com/bacteriophage-ndash-a-promising-alternative-measure-for-bacterial-biof-peer-reviewed-fulltext-article-IDR 1
https://www.frontiersin.org/articles/10.3389/fcimb.2018.00376/full 2
https://elifesciences.org/articles/16111 3
https://en.wikipedia.org/wiki/Phage_therapy 4
https://www.genengnews.com/insights/lysins-unlimited-phages-secret-weapon/ 5
https://www.intechopen.com/chapters/76569 6
https://academic.oup.com/femsre/article/33/4/801/583900 7
https://www.futuremedicine.com/doi/10.2217/fmb.12.97 8
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0060754 9
http://www.gladskin.com/


I would prefer lysins over whole phage........ Great achievement