In a recent report, the Science, Innovation & Technology Committee (SITC) has advocated for the UK government’s investment in advanced manufacturing plants dedicated to bacteriophages—viruses renowned for their bacteria-killing properties. This initiative seeks to address the diminishing efficacy of antibiotics against pathogens.
The SITC document recommends the reconsideration of repurposing the Rosalind Franklin Laboratory in the West Midlands, originally established for COVID testing but currently listed for sale. The proposal suggests transforming this facility into a manufacturing unit capable of producing phages for clinical trials, a move that could overcome a significant hurdle in bacteriophage research and development (R&D).
One primary obstacle in advancing bacteriophage studies to human trials is the requirement for production in a Good Manufacturing Practices (GMP) compliant facility. The SITC emphasizes that investment in such a plant should be contingent upon successful studies, aligning with the urgency to find alternatives to antibiotics.
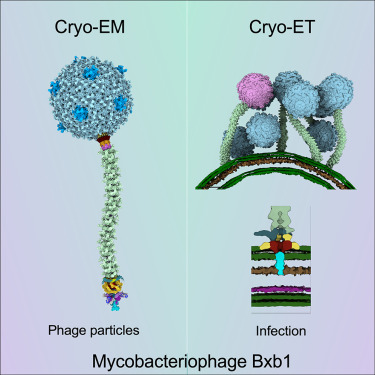
Though the potential of phages to combat bacteria has been acknowledged for over a century, their development waned in favor of antibiotics due to ease of testing and manufacturing. With antimicrobial resistance (AMR) rendering many antibiotics ineffective and a scarcity of new drugs in the pipeline, revisiting phages has become imperative.
Greg Clark MP, the Chair of the SITC, expressed astonishment at the proposed sale of the Rosalind Franklin Laboratory, which received over £1 billion in public funding and was intended to be a critical asset in national pandemic resilience. The committee’s report suggests repurposing the laboratory for bacteriophage production, preventing its loss to the nation and science through a firesale.

The report recommends the establishment of a small GMP facility modeled after the UK’s Catapult network, providing a resource for phage developers who might find the individual investment challenging. This approach aligns with the committee’s call for structural changes, urging regulators, policy-makers, researchers, and clinical practitioners to collaborate closely.
The urgency of this initiative is underscored by Professor Martha Clokie, the Director of the Centre for Phage Research at the University of Leicester. She points to a global crisis, with a rising number of patients succumbing to bacterial infections that current treatments cannot address. Recent statistics from the European Centre for Disease Prevention and Control estimate over 35,000 annual deaths from AMR across the EU and European Economic Area. Leicester recently opened UK’s first phage library.