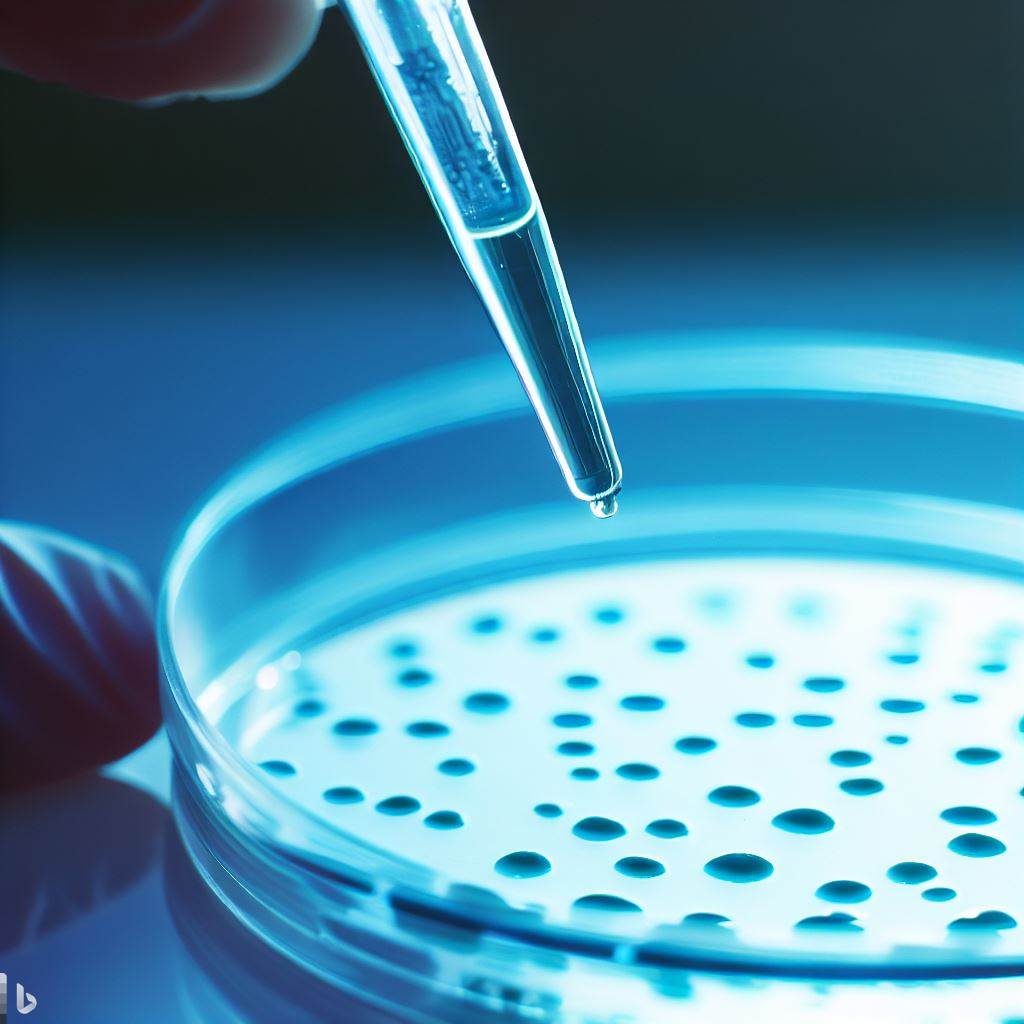
A spot test is a technique used to determine the presence and activity of bacteriophages against specific bacterial strains. It involves spotting a small volume of the phage solution onto a bacterial lawn, which is a confluent layer of bacterial cells grown on an agar plate. The spotted phage solution is allowed to diffuse and interact with the bacterial lawn under suitable incubation conditions. If the phages are active and capable of infecting the target bacteria, they will form visible plaques or lysis spots on the bacterial lawn, indicating the presence of phage-mediated bacterial cell lysis. The spot test provides a quick and qualitative assessment of phage activity against specific bacterial strains, making it a valuable tool in phage research and phage therapy applications.
What you need to perform a spot test
- LB (Luria-Bertani) agar plates: These are solid media plates used for culturing bacteria. The plates should be prepared in advance and allowed to solidify before use.
- 18-hour bacterial cultures: These are liquid cultures of the target bacterial isolates or reference bacterial strains grown in LB broth. The cultures should be prepared in sterile tubes or flasks.
- Micropipette: A micropipette is needed to transfer precise volumes of the bacterial culture and phage solution. In this case, you would need a micropipette capable of dispensing 500 µl and 10 µl volumes.
- Pipette tips: Disposable pipette tips are used with the micropipette for accurate and contamination-free liquid transfers. Make sure to use new tips for each transfer to prevent cross-contamination.
- Half-lid cover: This is a specific type of lid cover that partially covers the agar plate, allowing for better gas exchange while minimizing contamination risk.
- Incubator: An incubator is required to maintain a constant temperature of 37 °C for the bacterial lawns to grow and for the phage infection to occur. Ensure that the incubator is set at the appropriate temperature before starting the experiment.
- Timer or clock: You will need a timer or clock to accurately measure the incubation time of 20 minutes for the bacterial lawns and 6-18 hours for the detection of plaques or lysis spots.
- Appropriate safety measures: It is important to follow proper safety protocols, such as wearing gloves and lab coats, working in a sterile environment, and properly disposing of biological waste.
Note: Additional materials such as agar plates, LB broth, and phage solutions should be prepared and sterilized according to standard laboratory procedures before starting the experiment.
Procedure
- Prepare bacterial lawns of target bacterial isolates or reference bacterial strains by adding 500 µl of LB 18 h cultures on LA plates, allowing the liquid bacterial culture to soak into the LA, with a half lid cover on the plate, at room temperature or incubator at 37 °C for 20 min (Culture cells are healthy and grow rapidly, therefore, to prevent bacterial growth and too much thickening of the bacterial lawn, the plate should be used within 1 h at room temperature).
- Transfer 10 µl of the possible phage solution (this is the actual spotting) to the bacterial lawns and then incubate at 37 °C.
- Check for plaques or lysis spots that are observed after 6–18 h. The detection of phage presence is based on the visual appearance of a lysis zone at the site where the 10 µl solution was added onto the surface of the target bacterial lawn.
Results of the test
Positive results are expressed by either clear or semi-clear (turbid) lysis zones, while negative results are expressed by the absence of such lysis zones
For more protocols please visit The Phage protocols