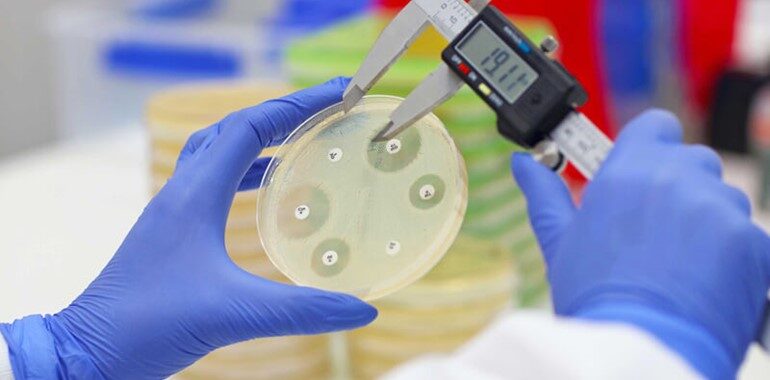
SNIPR Biome has conducted a randomized, double-blinded, placebo-controlled clinical trial. This is a Phase 1b clinical trial of SNIPR001 in patients with hematological cancer. These cancer patients, when treated with CRISPR armed phage therapeutic, showed positive results with successful target engagement. SNIPR001 was orally administered to 24 patients with hematological cancer.
Hematological cancers (blood cancers) are a group of cancers affecting the bone marrow and cells of the immune system. These patients suffer from bacteremia, mainly caused by Escherichia coli, leading to significant mortality. Most of these isolates are fluoroquinolone-resistant strains. Fluoroquinolones are a class of broad-spectrum antibiotics used to treat various bacteremia infections, further disrupting beneficial gut microbiota.
“The SNIPR001 combines four engineered phages (CAPs – CRISPR-CAS armed phages) that specifically target fluoroquinolone-resistant Escherichia coli in the gut microbiome. This precision approach was developed after screening wild-type phages against phylogenetically diverse Escherichia coli strains, followed by sophisticated engineering that incorporates both tail fiber modifications and CRISPR-CAS machinery to enhance targeting specificity and reduce resistance development, while preserving beneficial gut microbiota.”
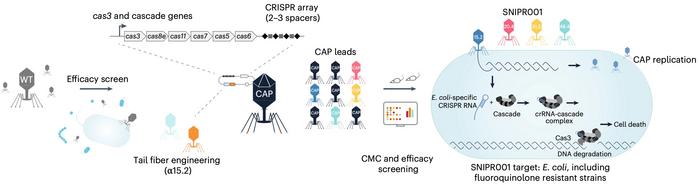
Dr Christian Grøndahl, Co-founder and CEO of SNIPR Biome, said, “This milestone builds upon the highly encouraging data from our CARB-X funded Phase 1a trial in healthy volunteers, where SNIPR001 demonstrated promising safety and target engagement.” SNIPR Biome is a CRISPR microbiome company; its main goal is to engineer microbiomes using CRISPR technology to treat several serious and life-threatening diseases.
Erin Duffy, Chief of Research & Development, CARB-X, said: “ The dosing of the first patient in this Phase 1b trial marks a significant step in evaluating a novel approach to preventing bloodstream infections caused by drug-resistant E. coli in vulnerable cancer patients. SNIPR001 represents a scientifically innovative strategy that could contribute to addressing an urgent clinical challenge.”
Read the press release here
For more phage-related global news updates, click here