Programmed cell death (PCD), sometimes termed apoptosis refers to any form of cell death mediated by an intracellular death program. Apoptosis is more precisely defined as a pathway that allows animals to efficiently eliminate damaged or excess cells while preserving metabolic resources and avoiding an immune response. However, apoptosis is not the only type of cell death. Plants, for example, maintain cell number homeostasis and initiate cell death as part of a wound response while containing no members of the metazoan apoptotic program. Furthermore, metazoans eliminate damaged or excess cells through necrosis and autophagy, both of which are genetically programmed. The consequences of these types of cell death are markedly different from those of apoptosis. PCD has also been found in unicellular eukaryotes and bacteria.
Bacteria are under constant attack from bacteriophages (phages), bacterial parasites that are the most abundant biological entity on earth, having both living and non-living characters. To resist phage infection, bacteria have evolved an impressive arsenal of anti-phage systems. Recent advances have significantly broadened and deepened our understanding of how bacteria battle phages, spearheaded by new systems like CRISPR-Cas.
Mechanism of abortive infection by bacteria against phage
Abortive infection (Abi) is a process by which cells prevent the release of functional phage virions at the expense of host cell survival/fitness. It is considered an altruistic action, a “programmed cell death” that prevents the spread of the phage to the surrounding clonal bacterial population. This is achieved by perturbating essential cellular processes such as translation, transcription, and replication or inducing membrane leakage. How phage infection is recognized to trigger the Abi response is often unknown.
Diagram showing abortive infection in bacteria
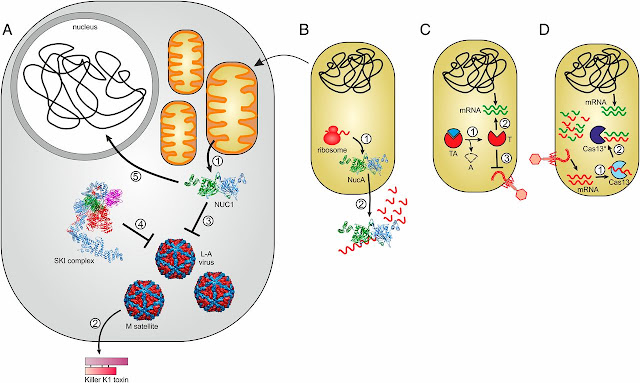 |
| Connections between immunity and programmed cell death photo courtesy by Koonin and Krupovic, 2019. |
A detailed explanation of Abortive infection from the diagram above
(A) Model of the NUC1-dependent antiviral defense. (1) NUC1 is secreted from mitochondria. (2) M satellite of totivirus L-A produces the secreted toxin K1, killing M-free yeast species. (3) NUC1 controls the L-A virus and M satellite propagation. (4) NUC1-mediated defense is redundant with the antiviral activity of the SKI complex (Ski2-3-8). (5) Concomitantly with the programmed cell death, NUC1 fragments the nuclear DNA. (B) Bacterial NUC1 homologs are dimeric secreted nucleases (1) involved in the degradation of extracellular nucleic acids (2). The arrow connecting A and B signifies the evolution of mitochondria from an α-proteobacterial ancestor and the likely inheritance of the NucA-like nuclease. (C) Role of toxin-antitoxin (TA) systems in programmed cell death and antiviral immunity. (1) Inactivation of labile antitoxin (A). (2) Upon virus infection, the toxin (T) is activated and induces cell dormancy or death by different mechanisms, for example, degradation of host mRNAs depicted in the figure. (3) As a result of toxin-induced cell dormancy/death, the virus cannot reproduce and spread in the population. (D) Cas13-mediated virus immunity and cell dormancy. (1) Upon virus infection, cognate CRISPR spacers targeting viral mRNA activate promiscuous RNase activity of Cas13. (2) Activated Cas13* indiscriminately degrades viral and cellular transcripts, leading to cell growth arrest so that the virus cannot reproduce and spread in the population.
Abortive cell death terminates the spread of infection from one cell to another, saving the population of bacteria from being infected by the prophages.