When people search “The Phage”, something funny happens: the first result is often a Star Trek storyline about a devastating fictional disease that wiped out the Vidiians. In that universe, The Phage is a highly adaptive illness that eats away at tissues, forces a society into desperate organ theft, and basically becomes one of the most feared conditions in the franchise. It’s dramatic, intense, and honestly a bit horrifying.
But here’s the thing:
That is not what “The Phage” means in real science.
In biology, a phage, short for bacteriophage, is something completely different. It’s not a disease. It’s not a plague. And it definitely doesn’t steal organs.
Phages are viruses that infect bacteria, and they’re actually some of the most abundant, elegant, and impactful biological entities on the planet.
So in this article, let’s clear the confusion. I’ll talk a bit about the Star Trek version, but then we dive deep into the real world of bacteriophages, how they work, why they matter, their role in the human gut, how scientists study them, and why the world is turning back to phage therapy as one of the most promising solutions to antibiotic resistance.
Let’s get into it.
What Actually Is “The Phage”?
In biology, “the phage” refers to bacteriophages, viruses that infect and replicate inside bacteria.
The word phage comes from the Greek phagein, meaning “to eat.”
Not because they literally chew on bacteria, but because they consume them from the inside out.
A quick breakdown:
- They attach to bacterial cells
- Inject their genetic material
- Hijack the host machinery
- Produce new virus particles
- Burst out (lytic cycle) or integrate into the genome (lysogenic cycle)
These processes shape ecosystems, human health, microbiomes, and even global nutrient cycles.
Quick Clarification:
Star Trek’s “Phage” = fictional disease
Real-world “phage” = bacteriophage (virus that kills bacteria)
The only thing they have in common is the name.
The Biology of Bacteriophages: How They Work
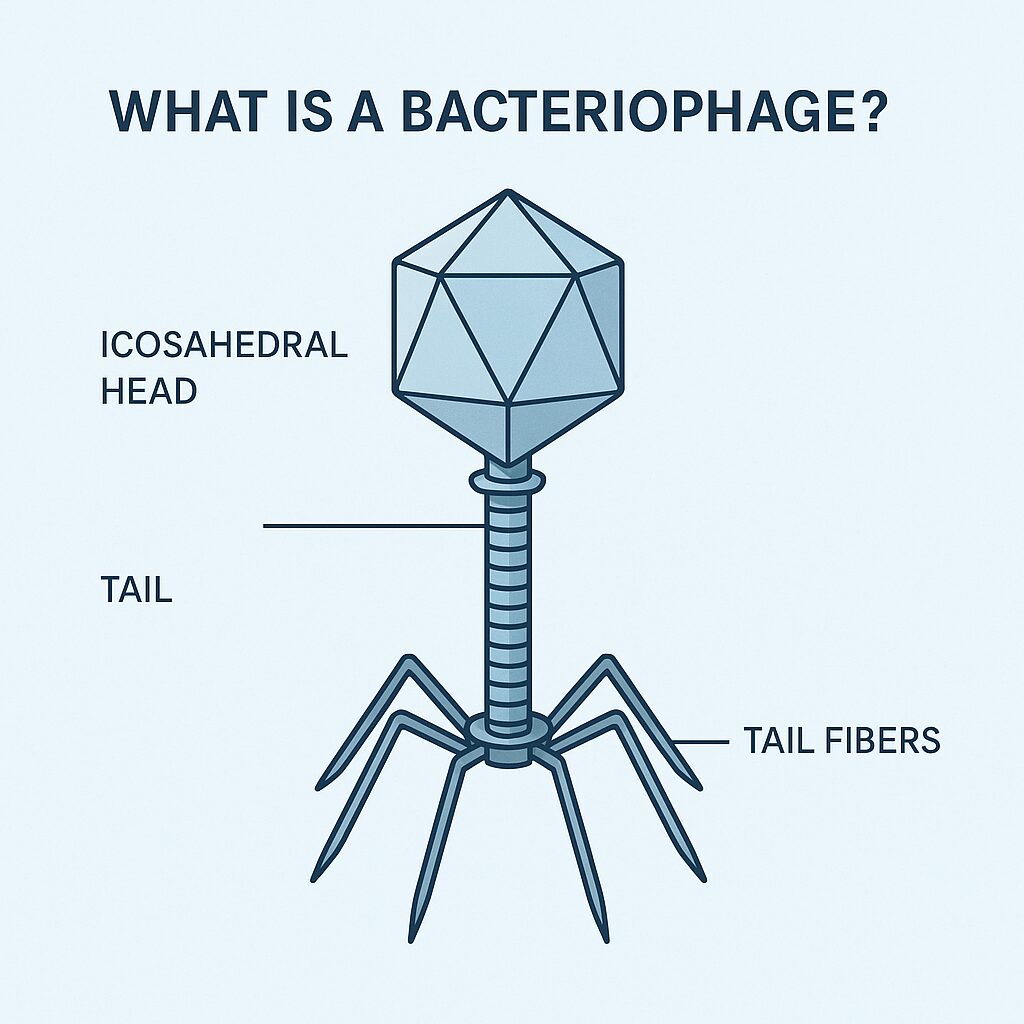
Phages are surprisingly complex for something so small. Their structure is almost iconic. Phages come in different shapes, but the most prevalent shape is T-phages.
In general, a T-phage will have:
- An icosahedral head containing DNA or RNA
- A protein tail used to latch onto bacteria
- Tail fibers that recognize specific bacterial species
They are often described as nature’s “nanomachines” because everything about them is built for precision.
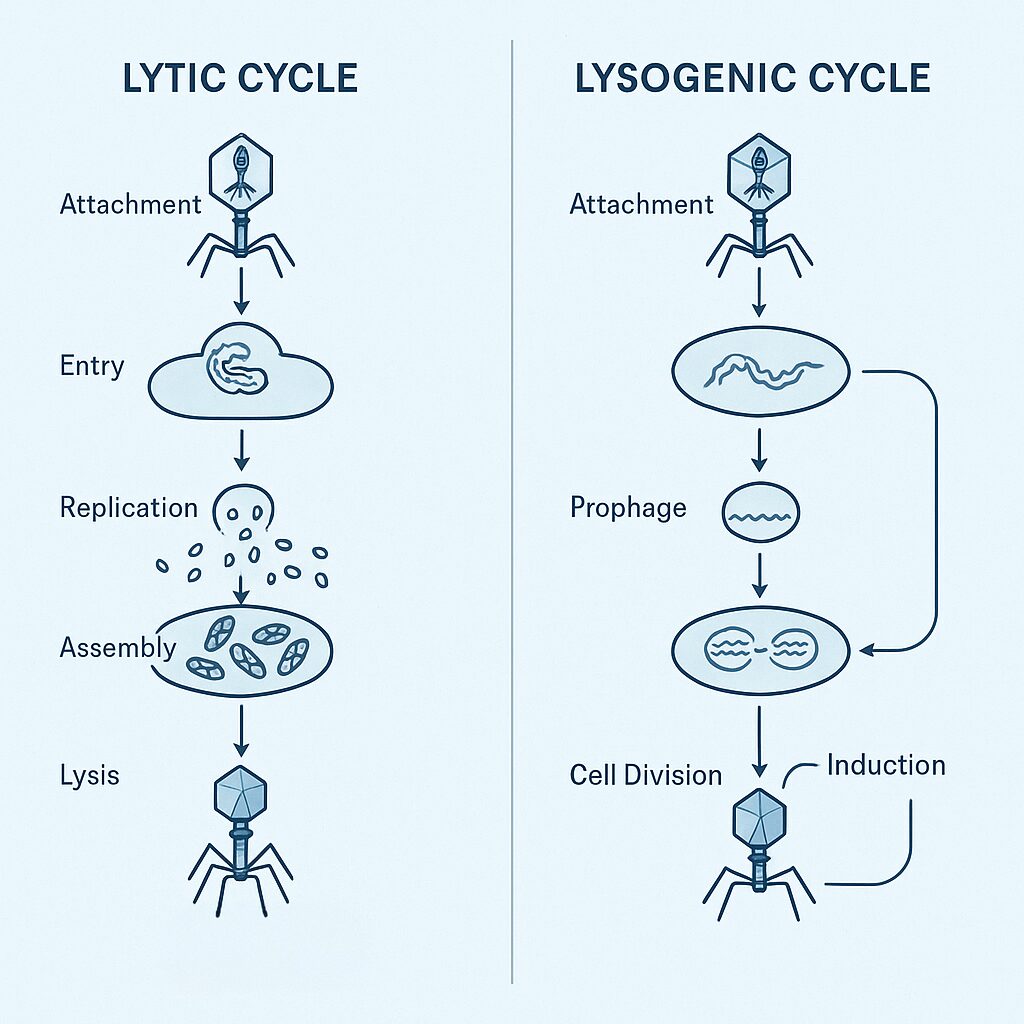
1. Lytic Phages (the bacteria killers)
These immediately begin replicating inside the host and eventually burst it open.
Outcome:
Fast and aggressive killing — perfect for phage therapy.
2. Lysogenic Phages (the genome integrators)
These integrate into the bacterial chromosome and stay silent for a while.
Outcome:
They influence bacterial evolution and behavior.
They can carry genes for toxins, immunity systems, or metabolic traits.
Together, these cycles shape the entire microbial world.
Why Phages Are Important for Modern Medicine
We are living in the age of antibiotic resistance. Bacteria are evolving faster than our drugs can keep up. But phages come with natural advantages:
They target specific bacteria
Unlike antibiotics that kill everything, phages are precise.
They evolve alongside bacteria
If bacteria resist them, phages adapt. Nature keeps the arms race going.
They are safe for humans
Phages don’t infect human cells.
They can reach places antibiotics struggle with
Biofilms, gut environments, complex microbiomes phages have access.
This is why phage therapy, first introduced in the early 1900s, forgotten in the antibiotic era, and now revived, is becoming a major global focus again.
The Phage in the Human Gut: Your Hidden Viral World
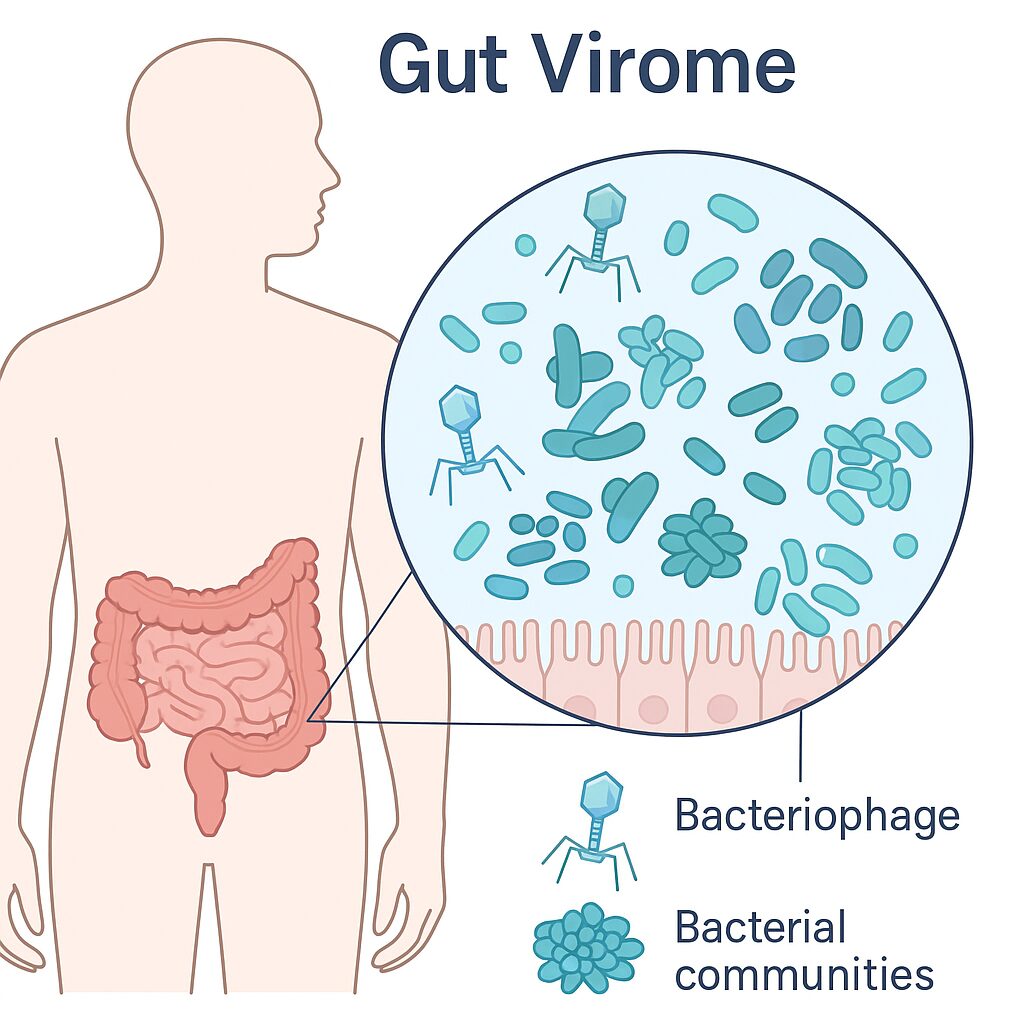
One of the most fascinating things we’ve learned in modern viromics research (and something I work on daily) is that your gut is packed with phages. The gut virome is a huge layer of invisible viral diversity that sits on top of your microbiome. Bacteriophages contribute to ~90% of viruses in your gut, and a change in proportion may be an indication of disease.
They do things like:
- Regulate bacterial populations
- Shape microbiome stability
- Influence nutrient metabolism
- Contribute to individuality — even people in the same household have unique viromes
- Trigger or prevent inflammation (active research area)
Some phages become stable members of your gut community. Others show up like storms. Understanding them helps us understand human health, aging, resilience, and disease.
Phages in the Environment
It’s impossible to talk about phages without appreciating their scale.
There are 10³¹ phages on Earth.
That’s more than all the stars in the universe.
They are everywhere:
- Oceans
- Soil
- Hot springs
- Your mouth
- Deep-sea vents
- Polar ice
They regulate global carbon cycling, bacterial turnover, and the evolution of microbial ecosystems.
How Scientists Study Phages Today
If you’re curious how we actually get these insights, here’s a breakdown of modern phage science:
1. Classical isolation
Plaque assays, enrichment cultures, host range experiments and phage microscopy.
2. Metagenomics
Sequencing all the DNA in a sample to identify viruses that cannot be grown in the lab.
3. vOTU clustering & viral taxonomy
Grouping viral genomes into species-level units.
4. Host prediction
Tools like
- WIsH
- geNomad
- Crispr matching
- k-mer similarity
- prophage linking
These methods help us understand which bacteria a phage infects, arguably the most important question in viromics.
5. Viral annotation
Using gene catalogs (PHROGs, VOGs, DRAM-v, etc.) to identify:
- tail fibers
- capsid proteins
- integration enzymes
- AMGs (auxiliary metabolic genes)
This is the modern toolkit for unlocking virome complexity.
The Future of Phage Research
We are entering a new era, and it’s exciting.
Coming advancements:
- Engineered phages
- Synthetic tail fibers for wider host range
- CRISPR-enhanced phages
- Personalized phage cocktails
- Virome-based therapeutics
- Phages as microbiome modulators
The world is realizing something important:
Leave a Reply
You must be logged in to post a comment.