Imagine being able to design a medicine so precise that it recognizes one single protein in your body and ignores everything else.
A treatment so specific it could stop a tumour in its tracks without disturbing healthy cells nearby.
That’s not science fiction anymore.
A team of researchers from Pompeu Fabra University (Barcelona) and Stanford University (California) has created a groundbreaking system to find and design such ultra-precise molecules, and it all starts with a virus that naturally infects bacteria.
A billion tests in one go
At the heart of the discovery is a clever use of bacteriophages, viruses that infect bacteria but are harmless to humans.
Scientists have long used these tiny biological machines as a way to test how different peptides, short chains of amino acids, interact with other molecules. In those interactions, we get useful features like treatment or detection of stubborn diseases like cancer. But this new study takes the technique to a whole new level.
The team built a system that allows them to screen up to a billion different peptides at the same time. Each peptide acts like a miniature key, searching for the exact protein “lock” it fits.
In this case, the researchers were interested in two very similar locks:
- Fibroblast Activation Protein alpha (FAPα), which fuels tumour growth in many cancers, and
- Dipeptidyl Peptidase 4 (DPP4), an enzyme central to controlling blood sugar in type 2 diabetes.
Both proteins share roughly 70% of their structure, which has caused serious problems for drug developers.
Some cancer drugs designed to block FAPα also hit DPP4 by mistake.
And some diabetes drugs targeting DPP4 can interfere with FAPα, or even with bacterial proteins in the gut, potentially disturbing the microbiome.
The challenge has been clear for years:
How do you build a molecule that can tell the difference between two nearly identical proteins and act only on one?
Building a better molecular key
The answer, it turns out, came from blending biology with chemistry.
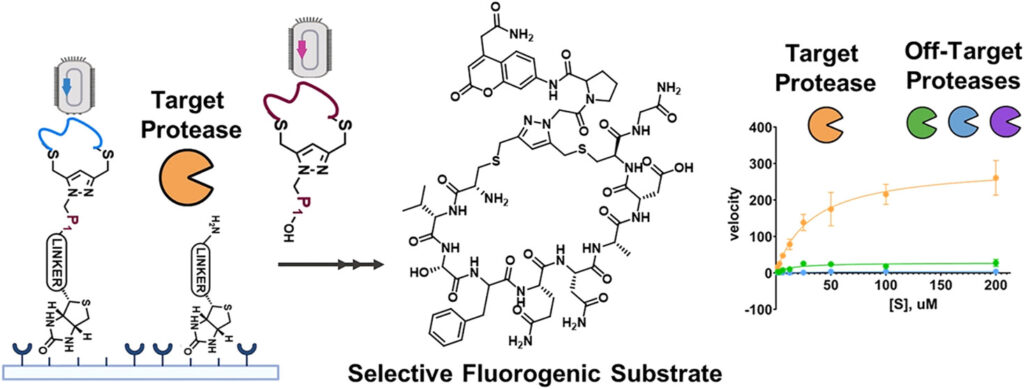
The researchers didn’t just rely on nature’s standard peptides. They created modified versions, adding special chemical structures that made each peptide into a small macrocycle, or ring-shaped molecule.
That circular shape gives the peptide rigidity and stability. It doesn’t bend or twist easily, which helps it fit only into the right protein site and avoid “false positives”, those random, unwanted interactions that cause side effects.
To make the process even more powerful, the team added a fluorescent molecule to every peptide. When the right key found its lock, it literally lit up, giving researchers a visual signal in real time.
The power of chemical precision
This combination of viral display, chemical modification, and real-time fluorescence created what scientists call a “phage display platform” — essentially a super-charged peptide discovery engine.
With it, the team could test enormous numbers of molecular candidates against both FAPα and DPP4. They then went through five rounds of selection, each time filtering out peptides that weren’t selective enough.
By the end of the process, they had a collection of peptides that could distinguish FAPα and DPP4 with incredible precision, some showing as much as 60-fold higher selectivity than commercial drugs or natural peptides.
To make sure these weren’t just lab curiosities, the scientists tested them in living cells. Even in the complex environment of a cell, where dozens of other enzymes are active, the peptides kept their focus, recognising only their intended targets.
From discovery to application
Once the team had identified these selective peptides, the next question was: what can we do with them?
The possibilities turned out to be wide-ranging and exciting.
Because these peptides are stable, selective, and fluorescent, they could be used not just as research tools, but as diagnostic agents or therapeutic building blocks.
1. Cancer imaging and guided surgery
In many cancers, FAPα is found at very high levels around tumours, up to 90% of carcinomas.
By attaching near-infrared fluorescent tags to these peptides, surgeons could someday use them to visually highlight tumour tissue during operations, ensuring that no malignant cells are left behind.
2. Safer treatments for diabetes
The selective peptides for DPP4 could serve as blueprints for next-generation diabetes drugs — ones that precisely target the human enzyme without accidentally binding to similar proteins in bacteria or the immune system.
That would reduce the chance of side effects and improve long-term metabolic balance.
3. New biomarkers for disease
Because these macrocyclic peptides can recognise specific proteins even in a crowded biological environment, they could help detect biomarkers, molecular warning signs, for early-stage cancers or metabolic disorders.
A faster, smarter way to find new medicines
Traditionally, finding selective molecules for a specific protein can take years and involve tedious trial-and-error experiments.
What makes this platform different is its speed and flexibility.
- The process is fully automated and quantitative thanks to the built-in fluorescent tracker.
- The same viral library can be chemically tweaked to target any protease, simply by modifying the linker molecule.
- And because it uses natural and non-natural amino acids, it explores a much wider chemical universe than traditional peptide screens.
This means scientists can now explore millions — even billions — of potential drug candidates in weeks instead of years, identifying those rare few that hit their targets with laser-like precision.
The bigger picture: toward “molecular personalization”
The implications go far beyond cancer and diabetes.
This kind of peptide design could be applied to diseases where proteases (enzymes that cut other proteins) play a role, from fibrosis and arthritis to Alzheimer’s and infectious diseases.
Even more, the technology could be adapted to develop theranostic agents — molecules that combine therapy and diagnostics in one. A peptide could, for example, light up a tumour for imaging and then deliver a targeted drug to it at the same time.
Looking ahead
The team is now working to refine their technique and test the most promising peptides in animal models. They’re also exploring collaborations to adapt the system for clinical diagnostics and image-guided surgery, where fluorescent peptides could help doctors identify diseased tissue in real time.
At Stanford, collaborators are developing versions that could include infrared dyes, invisible to the eye but visible to medical cameras, ideal for detecting deep tumours or internal inflammation.
Meanwhile, at Pompeu Fabra University, researchers are looking at how these selective peptides could help study protease activity inside living tissues, potentially revealing new therapeutic targets scientists didn’t even know existed.
A new era of molecular precision
For decades, drug discovery has been like trying to pick a lock with a bundle of keys, slow, uncertain, and full of trial and error.
This new technique feels more like using a smart key that knows exactly which lock it fits.
It’s chemistry and biology working in harmony, guided by design, not chance.
And if it fulfils its promise, it could redefine how we think about drugs altogether:
not as blunt instruments that “treat” diseases, but as finely tuned tools that speak the language of biology itself.
By teaching peptides to recognize the difference between nearly identical proteins, scientists are building the foundation for the next generation of medicine, precise, intelligent, and deeply personal.
A billion molecular experiments at once, all leading toward a single goal: treatments that work exactly where they’re needed, and nowhere else.Featured image from the study referenced below
Further reading: Faucher, F. F., Blažková, K., Lovell, S., Bertolini, M., Herrero-Bourdieu, J., Cosco, E. D., … & Barniol-Xicota, M. (2025). Macrocyclic Phage Display for Identification of Selective Protease Substrates. Journal of the American Chemical Society, 147(30), 26307-26318.
Leave a Reply
You must be logged in to post a comment.