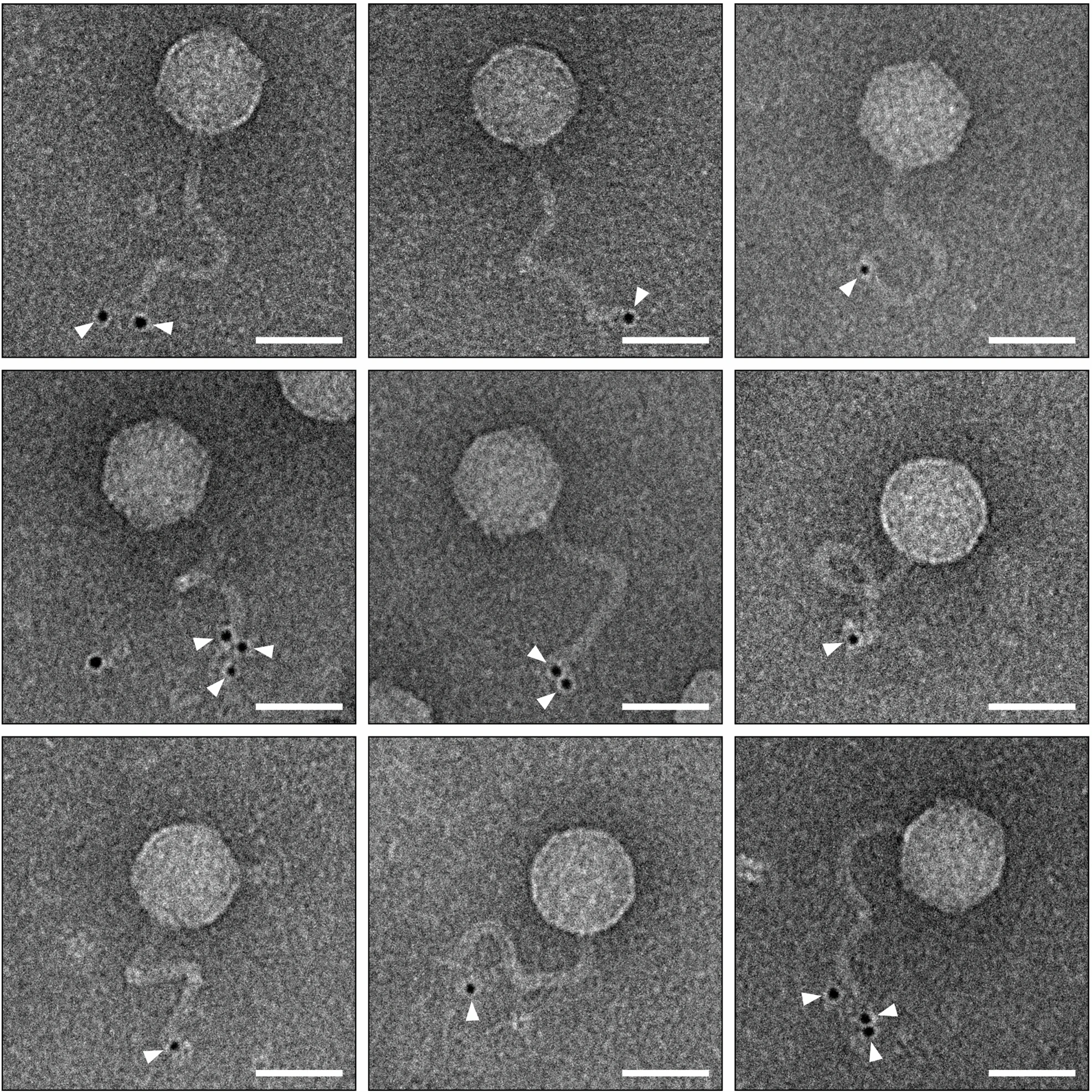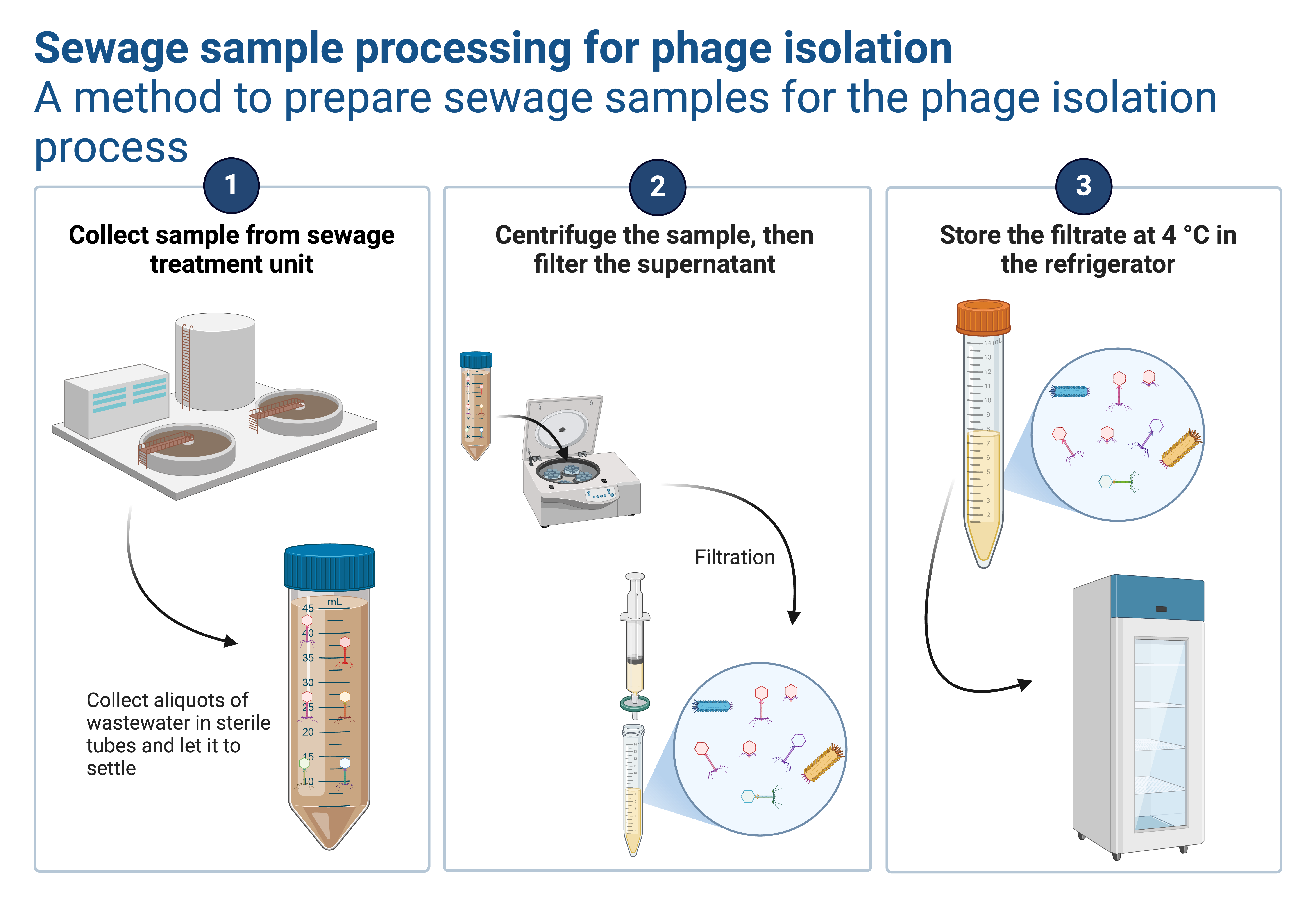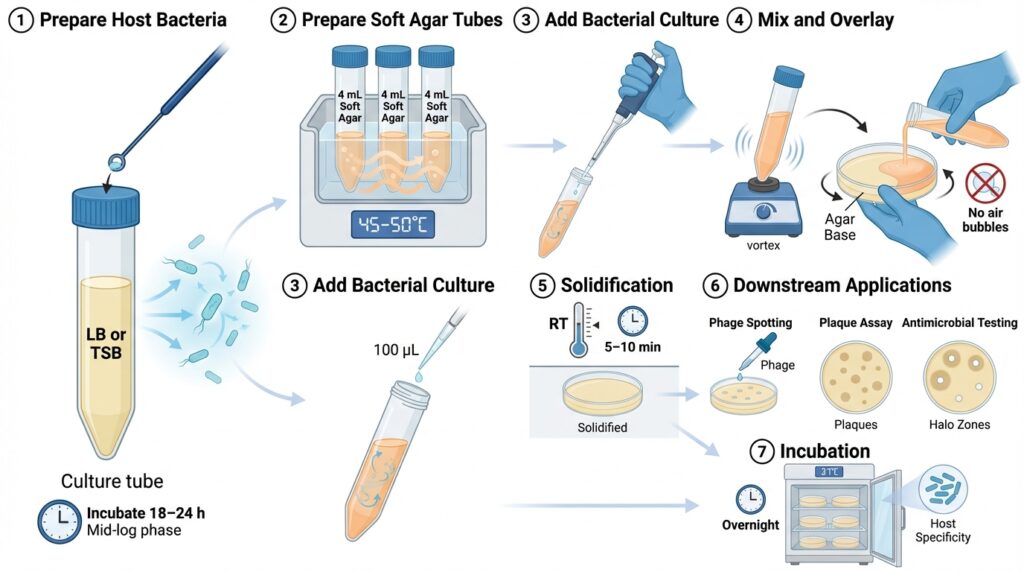
Overview
The agar overlay (double-layer agar) technique is a widely used microbiological method for generating a uniform bacterial lawn on solid media. It is essential for bacteriophage spot tests, plaque assays, phage amplification, and antibacterial activity assays.
In this method, host bacteria are suspended in soft (top) agar and poured over a solid base agar plate, creating a homogeneous lawn suitable for detecting zones of clearing or plaques.
Principle of the Method
Soft agar (typically 0.6–0.7% agar) remains semi-solid after setting, allowing bacteriophages to diffuse and infect nearby bacterial cells. When mixed with host bacteria and overlaid onto a solid agar base, the bacteria grow uniformly throughout the top layer.
Active bacteriophages lyse susceptible bacteria, forming clear plaques or zones of inhibition after incubation.
Applications
- Bacteriophage spot tests
- Plaque assays and phage titration
- Host-range determination
- Phage amplification
- Antibacterial or antimicrobial activity testing
Materials and Equipment
Equipment
- Sterile capped 13 × 100 mm tubes (or equivalent)
- Sterile pipettes (0.1–1.0 mL) or sterile pipette tips
- Water bath or heat block set to 45–50 °C
- Vortex mixer
- Bunsen burner or spirit burner (for aseptic technique)
Supplies
- Soft (top) agar (0.6–0.7% agar)
- Fresh host bacterial culture
- Pre-poured nutrient agar base plates
(e.g., LB agar, tryptone soy agar, or other suitable medium) - Disinfectant container (e.g., diluted Lysol or laboratory-approved disinfectant)
Preparation Before Starting
- Fully melt soft agar and hold at 45–50 °C.
(This temperature keeps agar liquid without harming bacteria.) - Ensure agar base plates are dry and at room temperature or slightly pre-warmed.
- Prepare a fresh bacterial culture:
- Overnight culture is acceptable for routine work
- Log-phase culture (OD₆₀₀ ≈ 0.3–0.6) gives optimal plaque clarity
Step-by-Step Protocol
1. Prepare Host Bacteria
Inoculate 3–4 mL of nutrient broth (LB or tryptone soy broth) with the desired host bacterium.
Incubate for 18–24 hours (or until mid-log phase if required).
2. Prepare Soft Agar Tubes
Dispense 4 mL of molten soft agar into sterile capped tubes.
Maintain tubes in a 45–50 °C water bath or heat block to prevent solidification.
3. Add Bacterial Culture
Add 100 µL (0.1 mL) of host bacterial culture to each tube of molten soft agar.
4. Mix and Overlay
- Vortex briefly to evenly suspend bacteria.
- Immediately pour the contents onto a pre-warmed agar base plate.
- Gently tilt and rotate the plate to distribute the overlay evenly.
- Avoid introducing air bubbles.
- Stop movement before the agar begins to gel.
5. Solidification
Allow plates to sit undisturbed for 5–10 minutes at room temperature until fully solidified.
6. Downstream Applications
Once solidified, plates are ready for:
- Phage spotting
- Plaque assays
- Antimicrobial testing
7. Incubation
Invert plates and incubate overnight at the appropriate temperature
(commonly 37 °C, depending on the bacterial host).
Results and Interpretation
After incubation:
- Clear plaques or zones indicate bacterial lysis or growth inhibition.
- Plaque size, clarity, and number reflect phage activity and concentration.
- Turbid plaques may indicate temperate phages or partial resistance.
Notes and Tips
- Do not allow bacteria to remain in molten agar for more than 1 minute before pouring.
- Agar temperatures above 50 °C may reduce bacterial viability.
- Overlay volume can be adjusted (typically 3–4 mL per 90 mm plate).
- Consistent timing improves reproducibility.
Safety and Waste Disposal
- Dispose of used materials in appropriate disinfectant or biohazard waste.
- Follow institutional biosafety guidelines for microbial handling.
Summary
The agar overlay technique is a core method in bacteriophage research, enabling reliable visualization of phage–host interactions. When performed correctly, it produces reproducible bacterial lawns suitable for high-quality phage assays.