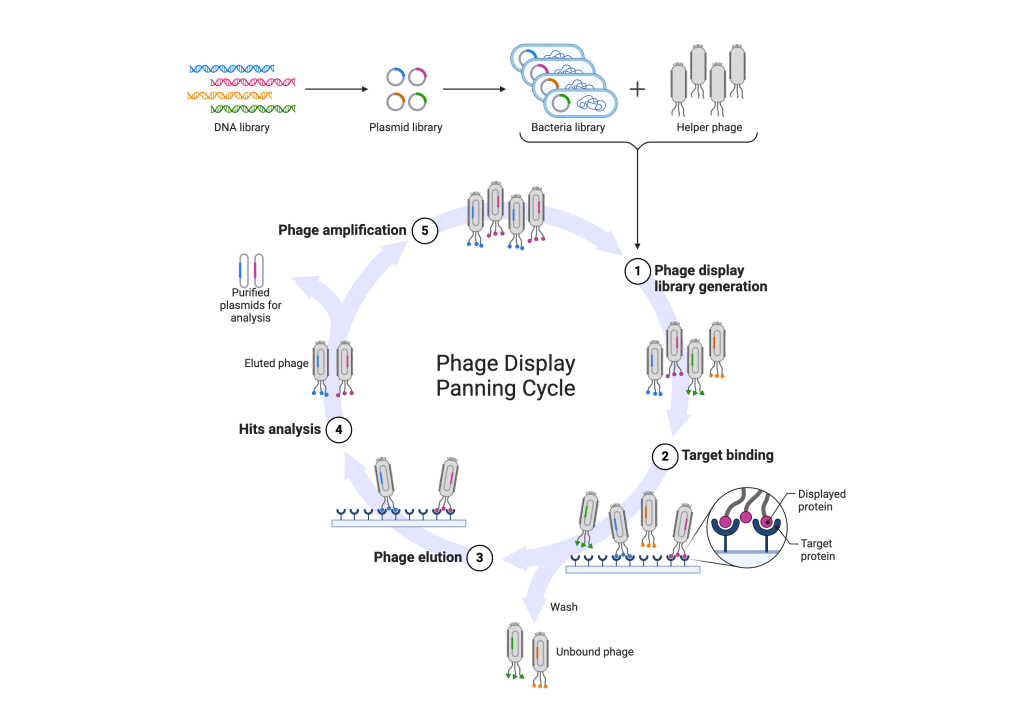
Extracting the DNA from a bacteriophage is essential in order to study its genetic makeup and identify the genes that encode its various functions. There are many methods that can be used to extract DNA from a bacteriophage, but one of the most commonly used methods is called the SDS-proteinase K method. Many companies have come up with kits that ease the extraction of genetic materials from bacteriophages. The following protocol is about DNA extraction using the miniprep protocol. This produces enough DNA for subcloning and mapping the fragments in about 30 min.
You need to have 1.5 ml (or more) of a very high titer liquid lysate that has been cleared with chloroform. This step should follow after isolation, purification, and bulking up steps.
Materials:
TES: 0.1M Tris-HCl pH 8.0 0.1M EDTA 0.3% SDS
Method:
- Add DNAse and RNAse to a final concentration of 100 ug/ml and incubate at 37oC for 30 min.
- Add 20 ul of filter sterilized 2.0 M ZnCl2 to each 1 ml of lysate and incubate for 5 min at 37o
- Centrifuge for 1 min and remove supernatant. There should be a substantial pellet containing the phage at the bottom of the tube.
- Resuspend the pellet in 500 ul of TES with the help of a tip or sterile toothpick and incubate at 65o for 15 min.
- Add 60 ul 3M potassium acetate pH 4.8 (standard alkaline lysis miniprep solution). Mix thoroughly and incubate on ice for 15-20 min.
- Centrifuge for 1 min and remove supernatant to a new tube.
- Add an equal vol of isopropanol, mix and incubate on ice for 5 min.
- Centrifuge for 10 min, wash the pellet with 70% EtOH and dry. Resuspend in an appropriate volume of water or TE
Notes:
- The necessity of filter sterilizing the ZnCl2 is still questionable.
- The ZnCl2 does "go bad" as the duration of the solution storage increases. Therefore, It's advised to prepare it in small batches ie 1-5 ml. (only use about 20 ul at a time!)
- The very retentive may want to add a phenol: chloroform extraction between steps 6 and 7!

Find more protocols by clicking here