Antimicrobial resistance (AMR) is one of the most pressing global health challenges of our time. Researchers from Monash University and The Alfred Hospital have developed Entelli-02, a bespoke phage therapy specifically designed to combat Enterobacter cloacae complex (ECC) infections, a group of bacteria that is increasingly resistant to antibiotics.
This innovative therapy represents a major advancement in precision medicine and hospital-specific treatments for drug-resistant infections.
What is Entelli-02?
Entelli-02 is a five-phage cocktail designed using a decade of bacterial isolates. The therapy works by targeting the specific bacteria causing infections, drastically reducing bacterial loads by over 99% in preclinical trials.
A Precision Approach to Treating Drug-Resistant Infections
The study, published in Nature Microbiology, was led by Professor Jeremy J. Barr from Monash University, School of Biological Sciences, with Professor Anton Peleg from the Department of Infectious Diseases at The Alfred. This development marks a new approach for precision medicine in hospitals battling antimicrobial resistance(AMR).
Development Process: From Lab Concept to Clinical-Grade Therapy
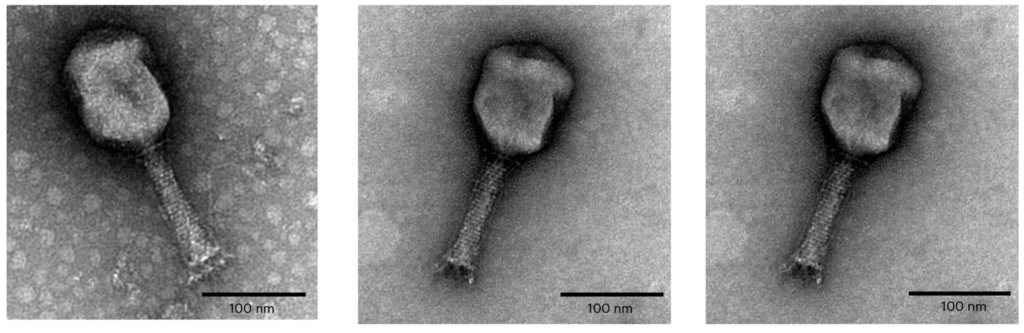
The development of Entelli-02 involved a decade’s worth of bacterial isolates. The research team, led by Dr Dinesh Subedi, developed and produced Entelli-02 through a rigorous process of phage isolation, genetic adaptation, and preclinical testing.
Initially, the team began by combining three phages into a cocktail. Through iterative design, they improved the cocktail by genetically adapting the viruses to expand their host range, followed by the selection of two additional phages with improved treatment outcomes. The final product contains five phages that could kill a broad range of Enterobacter isolates and reduce bacterial loads in infected mice by over 99%.
Manufacturing and Regulatory Approval
Entelli-02 was manufactured as a therapeutic-grade phage product at the Monash Phage Foundry, meeting sterility and safety standards for intravenous use under Australia’s Therapeutic Goods Administration (TGA) Special Access Scheme.
Professor Barr emphasized, “This is a blueprint for how hospitals can respond to AMR outbreaks with precision therapies. We’re bridging the gap between broad-spectrum antimicrobial treatments and personalized phage therapy to deliver a ready-to-use solution that’s both targeted and scalable.”
Why Bespoke Phage Therapy Matters
Traditional antibiotics are increasingly ineffective against AMR infections. Bespoke phage therapy offers:
- Precision targeting of specific bacterial strains
- Alternative treatment options when antibiotics fail
- Rapid development for hospital-specific outbreaks
- Potential to combat global antimicrobial resistance
This makes Entelli-02 a game-changer in infectious disease management and a promising tool in precision medicine.
Availability and Future Implications
Entelli-02 is now available for compassionate use, setting the stage for future clinical trials utilising phage products. The team hopes this hospital-specific phage cocktail model can be replicated in other hospitals facing similar AMR threats.
Reference: Subedi, D., Gordillo Altamirano, F., Deehan, R. et al. Rational design of a hospital-specific phage cocktail to treat Enterobacter cloacae complex infections. Nat Microbiol (2025). https://doi.org/10.1038/s41564-025-02130-4.
For more phage-related interesting reads, check here


Leave a Reply
You must be logged in to post a comment.