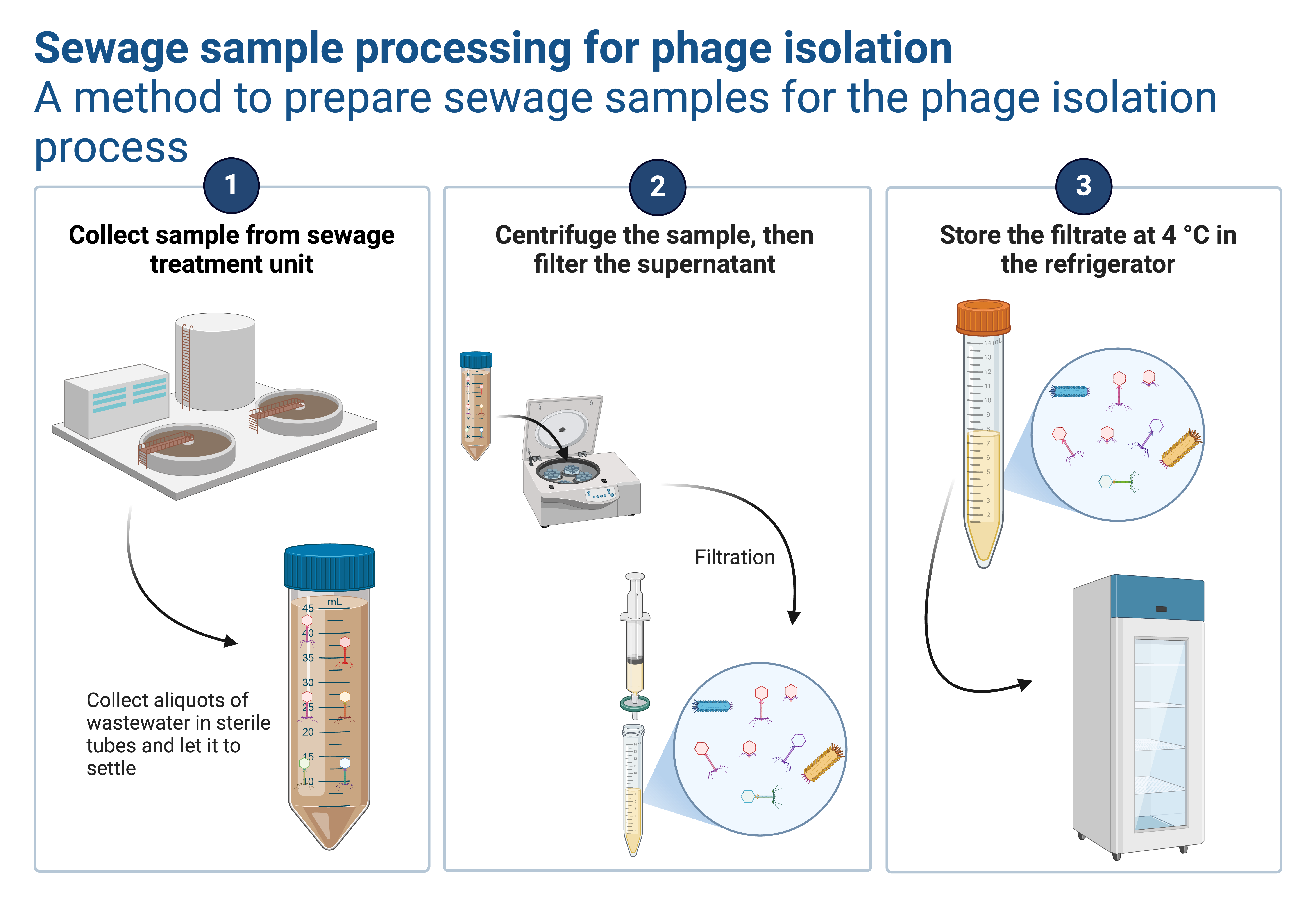
Purpose
Concentrate bacteriophages from a cleared lysate using PEG (polyethylene glycol) + NaCl, then resuspend as a smaller-volume, higher-titer phage prep.
Safety
Work with the appropriate biosafety level for your host bacterium and sample source. Use PPE and disinfect surfaces (e.g., 10% bleach followed by ethanol).
Materials
- Cleared phage lysate (plate lysate or liquid lysate)
- NaCl (to 1.0 M final)
- PEG 8000 (typical final 10% w/v)
(PEG 6000 can also work; PEG 8000 is most common) - Sterile SM buffer or PBS (for resuspension)
- Classic SM: 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 8 mM MgSO₄, 0.01% gelatin (optional)
- Centrifuge tubes (50 mL, 250 mL, etc.)
- Centrifuge (4 °C preferred)
- 0.22 µm filter (optional but recommended)
- Gentle rocker/rotator
Before you start (key notes)
- Start with a well-cleared lysate: remove cells and debris first, or PEG will co-precipitate junk.
- PEG precipitation concentrates phage, but may also bring down proteins/vesicles—downstream cleanup (chloroform extraction, DNase/RNase, CsCl, ultrafiltration) is optional depending on your goal.
Step 1 — Clarify the lysate
- Centrifuge lysate 10,000 × g, 10–15 min, 4 °C.
- Transfer supernatant to a clean tube.
- (Recommended) Filter through 0.22 µm to remove remaining bacteria.
Step 2 — Add NaCl (1.0 M final)
- Add NaCl to 1.0 M final.
- Example: for 100 mL lysate, add ~5.84 g NaCl (since 1.0 M NaCl ≈ is 58.44 g/L).
- Mix until fully dissolved.
- Incubate on ice or at 4 °C for 30–60 min.
- Centrifuge 10,000 × g, 10 min, 4 °C to pellet precipitated proteins/debris.
- Transfer the supernatant to a new tube.
Step 3 — Add PEG 8000 (10% w/v final)
- Add PEG 8000 to 10% (w/v) final.
- Example: for 100 mL lysate, add 10 g PEG 8000.
- Mix thoroughly (PEG dissolves slowly; stir/rock at room temp briefly if needed, then cool).
- Incubate at 4 °C for 2 hours to overnight (overnight usually gives the best yield).
Step 4 — Pellet the phages
- Centrifuge 10,000–12,000 × g, 20–40 min, 4 °C.
- Carefully decant the supernatant (PEG-containing) without disturbing the pellet.
- The pellet can be transparent/whitish and slippery—easy to lose.
(Optional wash)
3. Add 1–2 mL cold SM along the wall, gently swirl, and remove to reduce PEG carryover.
Step 5 — Resuspend the phage pellet
- Resuspend pellet in SM buffer/PBS:
- Typical: 1/50 to 1/100 of the starting volume
(e.g., 100 mL → resuspend in 1–2 mL).
- Typical: 1/50 to 1/100 of the starting volume
- Let sit at 4 °C for 30–60 min (or gentle rocking) until fully dissolved.
- Optional: brief spin 5,000 × g, 5 min to remove insoluble material; keep supernatant.
Step 6 — Storage
- Short-term: 4 °C (days–weeks depending on phage)
- Long-term: -80 °C with cryoprotectant (commonly 10–20% glycerol), or store as lysate at 4 °C if stable.
- Avoid repeated freeze–thaws.
Quality checks (recommended)
- Titer before and after (plaque assay) to estimate recovery.
- If you need cleaner prep:
- Dialysis (SM buffer) to remove PEG/salt
- Ultrafiltration (e.g., 100 kDa cutoff)
- Chloroform extraction (some phages are chloroform-sensitive—test first)
Troubleshooting
- Low recovery: incubate longer with PEG (overnight), ensure PEG and NaCl reach final concentrations, keep everything cold, and avoid losing the pellet.
- Pellet won’t dissolve: add more SM, resuspend gently, let sit longer at 4 °C with rocking.
- Downstream inhibition (PCR/enzymes): remove PEG by dialysis/ultrafiltration; PEG carryover is common.
- Phage inactivation: some phages don’t like high salt/PEG or long cold incubations—reduce incubation time or try milder PEG % (8%).
After precipitation next stage is usually centrifugation under Ceasium chloride (CsCl) gradient