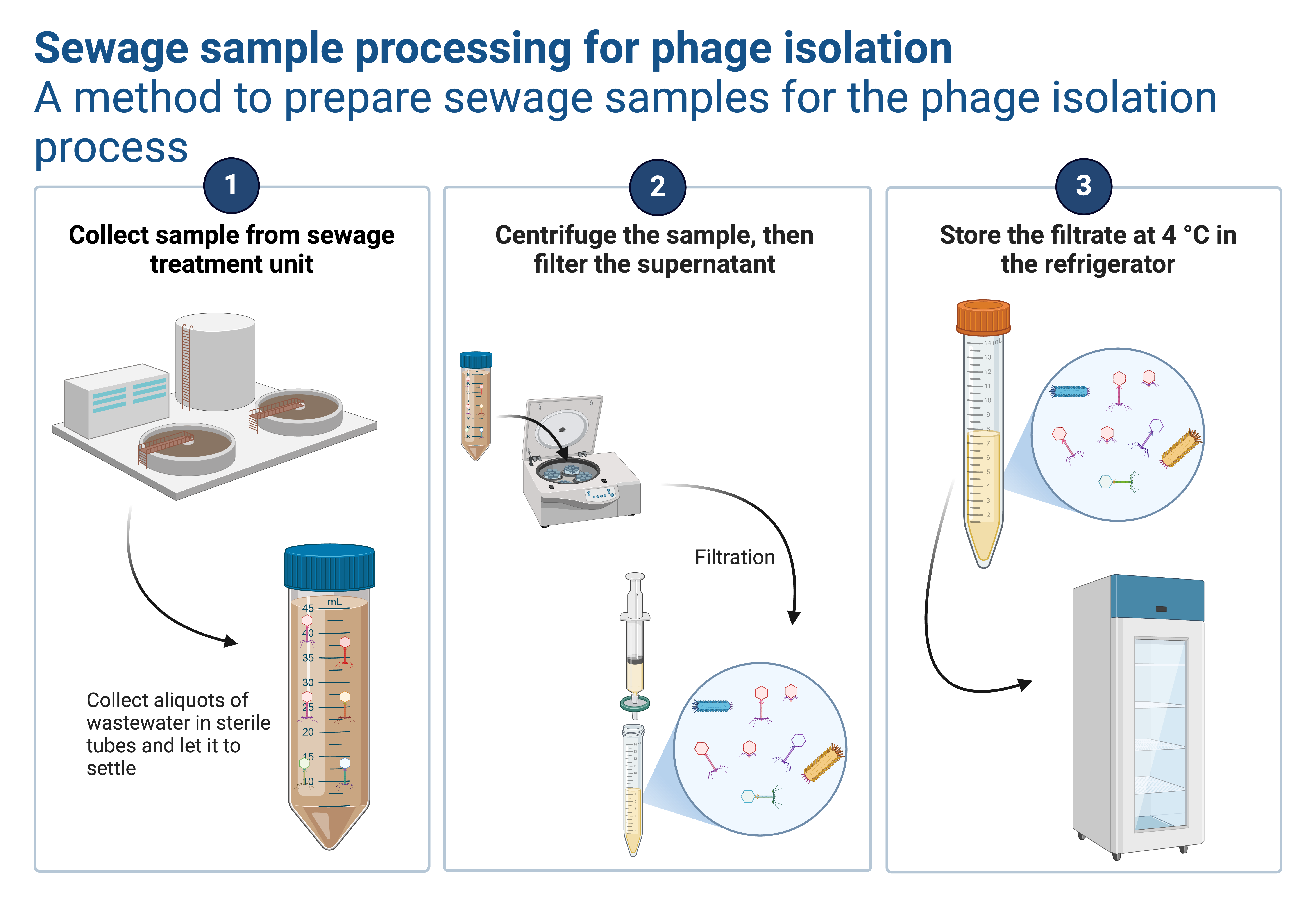
Overview
After PEG precipitation, bacteriophages can be further purified using a caesium chloride (CsCl) density gradient, which separates intact virions from host debris, membrane vesicles, and empty capsids based on buoyant density. This step yields highly purified phage preparations suitable for microscopy, proteomics, genomics, and structural studies.
Biosafety and handling notes
- CsCl is toxic: handle with gloves, a lab coat, and eye protection.
- Use ultracentrifuge-rated tubes and follow rotor-specific safety guidelines.
- Some phages lose infectivity in CsCl; minimise exposure time and dialyse promptly.
Materials
- PEG-precipitated phage pellet (resuspended in SM buffer)
- CsCl (ultrapure)
- SM buffer (or equivalent phage buffer)
- DNase I and RNase A (optional)
- Ultracentrifuge tubes compatible with your rotor
- Swinging-bucket or fixed-angle ultracentrifuge rotor
- Syringe with 18–23G needle or fraction collector
- Dialysis tubing or cassette (10–50 kDa MWCO)
Pre-gradient preparation
- Ensure the PEG pellet is fully resuspended in the SM buffer.
- Clarify by centrifugation at 5,000 × g for 5 min to remove insoluble material.
- (Optional) Treat with DNase I (1–5 µg/mL) and RNase A (1–10 µg/mL) for 30–60 min at room temperature to remove free nucleic acids.
- Keep samples at 4 °C until loading.
CsCl step gradient setup
CsCl gradients can be prepared as step gradients, which are simple and robust for routine phage purification.
Typical CsCl densities
Most tailed dsDNA phages band between 1.45–1.52 g/mL, but this can vary.
Prepare CsCl solutions in SM buffer at 4 °C:
- 1.30 g/mL
- 1.45 g/mL
- 1.50–1.70 g/mL (bottom layer; adjust based on phage type)
Use a refractometer to confirm densities whenever possible.
Gradient layering
- Add the highest-density CsCl solution to the bottom of the ultracentrifuge tube.
- Carefully overlay the intermediate density layer.
- Add the lowest-density layer on top.
- Gently overlay the resuspended PEG-precipitated phage sample.
Avoid mixing layers by pipetting slowly along the tube wall.
Ultracentrifugation
- 100,000–150,000 × g
- 2–4 hours
- 4 °C
Swinging-bucket rotors typically produce sharper, horizontal bands.
Phage band recovery
- After centrifugation, identify the opalescent band corresponding to intact phage particles.
- Collect the band using:
- Side puncture with a sterile needle, or
- Gentle top-down fractionation
- Transfer the recovered fraction into a sterile tube.
Removal of CsCl
Complete removal of CsCl is essential for downstream applications.
Dialysis
- Transfer phage fraction into dialysis tubing or cassette.
- Dialyse against large volumes of SM buffer at 4 °C.
- Perform 3–4 buffer changes over 6–24 hours.
Alternative buffer-exchange methods (e.g., centrifugal concentrators) may be used if compatible.
Final cleanup and storage
- Centrifuge dialysed sample at 5,000 × g for 5 min to remove precipitates.
- Store purified phage:
- 4 °C for short-term use
- −80 °C with 10–20% glycerol for long-term storage (if phage is freeze-tolerant)
Expected outcomes
- Highly purified, debris-free phage preparation
- Suitable for:
- Electron microscopy
- Proteomics and structural studies
- High-quality DNA extraction
- Reference phage stocks
Troubleshooting
- No visible band: insufficient starting titer; concentrate lysate further before CsCl.
- Multiple bands: empty capsids or membrane vesicles; collect the dominant band only.
- Low infectivity: reduce CsCl exposure time and dialyse immediately after collection.