If you have cystic fibrosis (CF), you hear the same name again and again: Pseudomonas aeruginosa is a stubborn bacterium that loves to move into the lungs and never leaves without a battle.
It doesn’t just float around freely. It builds biofilms, slimy clumps of bacteria glued together and wrapped in mucus. Inside those clumps, Pseudomonas becomes hard to reach with antibiotics and very good at surviving long-term.
Now imagine if we could teach viruses that infect bacteria (phages) to become better hunters inside those clumps.
That’s exactly what a new study in Nature Communications did: the researchers literally trained a virus to get better at finding and killing Pseudomonas in a CF-like environment.
First, what are phages?
Phages are viruses that only infect bacteria – they don’t infect human cells.
They work like tiny guided missiles:
- They land on a bacterial cell.
- Inject their DNA.
- Use the bacterial machinery to make more phages.
- Burst the cell open to release the next “generation” of virus.
Because of this, phages are being explored as living antibiotics, especially when normal drugs stop working.
In CF lungs, phages are exciting because they might:
- Get into thick mucus and biofilms
- Reach bacteria hiding from antibiotics
- Kill drug-resistant strains
But… It’s not that simple.
Why Pseudomonas biofilms are so hard to treat
Inside a CF lung, Pseudomonas doesn’t behave like a neat laboratory culture. It forms dense bacterial clumps and constantly changes its surface. One of the main structures on that surface is called LPS (lipopolysaccharide) – think of it as part of the outer “skin” of the bacteria.
Different cells in the same biofilm can have slightly different LPS designs. That means:
- Some cells are easy for a phage to grab onto.
- Others are basically “invisible” to the same phage.
So you can throw a phage at the biofilm and:
- Kill the easy targets
- Accidentally select for the “invisible” ones, which then grow back
This is one of the reasons phage therapy can sometimes reduce bacteria but not fully clear a biofilm.
The idea: let the phage learn inside the biofilm
Instead of designing a phage on a computer, the researchers tried something beautifully simple and evolutionary:
Let the phage evolve directly in the environment where we need it to work – a Pseudomonas biofilm from a CF patient.
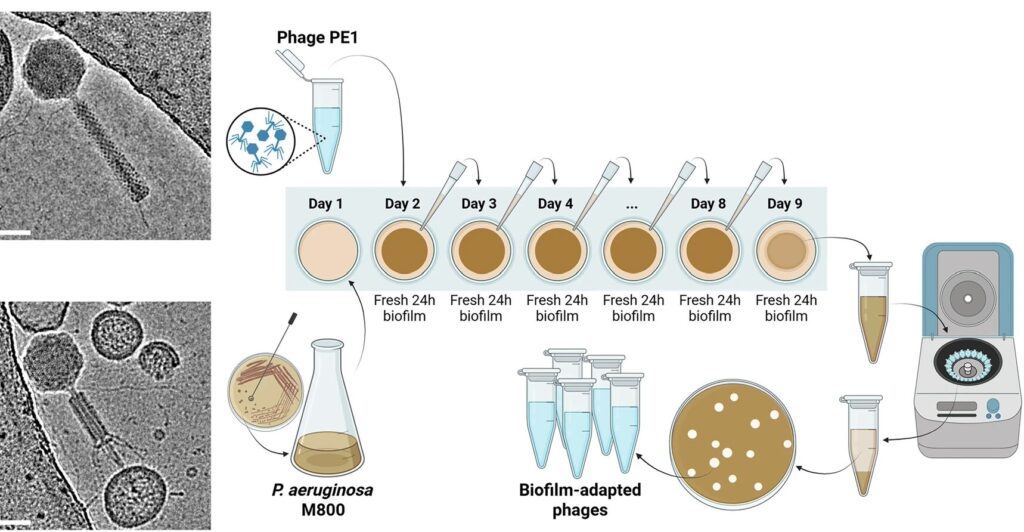
They started with:
- A phage called PE1, which infects Pseudomonas
- A Pseudomonas strain (M800) taken from a CF patient, which forms very dense clumps and is not very sensitive to the original phage
Then they did a kind of training bootcamp:
- Grow a fresh Pseudomonas biofilm for 24 hours.
- Add the phage and let it attack the biofilm for 24 hours.
- Collect the phages that made it through.
- Use those phages to infect a new, fresh biofilm.
- Repeat this cycle for 8 days.
Each round, only the phages that managed to find and infect bacteria in that messy, real-world biofilm were carried forward. That’s natural selection doing the hard work.
At the end, they picked a few of these “biofilm-trained” phages and tested them.
What changed after evolution?
Two evolved versions stood out: PE1-3 and PE1-5.
When the researchers compared them to the original PE1, they saw:
- On simple lab plates, the evolved phages made clearer, stronger plaques on the CF strain.
- In biofilms (including in a mucus-like CF medium), the evolved phages:
- Broke up bacterial clumps more visibly
- Reduced bacterial counts more than the original phage
They also tested the phages in a 3D lung cell model – human lung cells grown in a way that mimics real tissue and then infected with Pseudomonas:
- One of the evolved phages, PE1-5, was able to reduce the bacteria attached to the lung cells,
- And importantly, it didn’t harm the lung cells.
So the phage didn’t turn into a monster – it stayed specific to the bacteria but became better at controlling them in a realistic setting.
Under the hood: how did the phage get better?
When they sequenced the phage genome, the changes were surprisingly small:
- Just a few single-letter mutations in genes that code for:
- The baseplate (the part that helps anchor the tail to the virus)
- The tail fibres (the “legs” that actually recognise and grab the bacteria)
You can think of tail fibres like fingers feeling for a door handle. Those tiny mutations seem to have changed the “shape and stickiness” of those fingers in a useful way.
Here’s what that achieved:
1. Faster binding
In lab tests:
- The evolved phages stuck to bacteria much faster than the original one.
- Under the electron microscope, there were more evolved phages attached per bacterial cell.
So once they bumped into a bacterium, they were better at grabbing on and not letting go.
2. Recognising more “types” of Pseudomonas
Remember the LPS “skin” on Pseudomonas? It has different parts, including a long sugar chain called the O-specific antigen (OSA).
- The original phage relied heavily on that OSA to infect.
- If bacteria chopped off or altered that region, the phage struggled.
- The evolved phages learned to use another part of LPS – the core region – as an extra “handle”.
Many CF isolates, including their M800 strain, don’t make full OSA chains. That’s one reason the starting phage wasn’t very effective. By evolving in that environment, the phage essentially learned:
“Don’t just grab the fancy decorations (OSA). Also learn to hold onto the sturdy frame underneath (the LPS core).”
That shift is huge, because it means:
- The phage can now infect bacteria with full OSA,
- And bacteria that have shortened or lost those OSA chains – the ones that often pop up in chronic CF infections.
The bacterial side: evolution fighting back
While the phage was evolving, so were the bacteria.
The researchers looked at dozens of individual colonies from untreated biofilms and from biofilms exposed to the original phage.
They saw:
- Untreated biofilms contained a mix of variants, some with full OSA, some with shortened versions.
- After treatment with the original phage, the survivors were mainly the variants with truncated OSA, which the original phage couldn’t handle well.
This is classic evolutionary “arms race” behaviour:
- Phage targets bacteria with full OSA
- Bacteria with damaged or missing OSA survive and spread
- New phages that can recognise the LPS core instead suddenly have a big advantage.
By evolving in the biofilm, PE1-3 and PE1-5 effectively joined the next round of that arms race.
Why this matters for people with CF
This is still lab work – not a treatment you can get in a clinic yet – but it points to a very promising direction for phage therapy:
- Phages can be customised by evolution, not just by design.
Instead of guessing which mutations might work, we can let the biofilm itself select the best phages. - Adapting phages in realistic conditions is key.
Evolving a phage in a simple liquid culture might not prepare it for:- Thick mucus
- Dense aggregates
- Mixed bacterial subpopulations
Biofilm-based evolution keeps all those pressures “on” during training.
- We can target the real problem: diversity inside infections.
In CF, there isn’t just “one Pseudomonas”. There are many variants coexisting in the same lung. A good therapeutic phage (or phage cocktail) needs to hit as many of them as possible. - Small genetic tweaks can make a big clinical difference.
The fact that a handful of mutations in tail fibres and baseplate proteins can shift what LPS forms a phage recognises gives researchers very concrete targets if they ever want to move towards rational engineering on top of evolution.
So, are we ready to deploy “biofilm-trained” phages in patients?
Not yet – but this is a serious step forward.
What still needs to happen:
- Testing these evolved phages in animal models of CF lung infection.
- Understanding how they behave when combined with antibiotics or mucus-modifying drugs.
- Checking safety and stability in more complex systems.
But the concept is powerful and simple:
Instead of asking, “Why don’t phages work well enough in CF biofilms?”
this study asks, “What happens if we let the phages learn how to live and hunt there?”
And the early answer seems to be:
They do get better.
Source: Meneses, L., Valentová, L., Santos, S.B. et al. Directed evolution of phages in biofilms enhances Pseudomonas aeruginosa control through improved lipopolysaccharide recognition. Nat Commun 16, 10219 (2025). https://doi.org/10.1038/s41467-025-65014-5
Cover image credit Meneses et al


Leave a Reply
You must be logged in to post a comment.