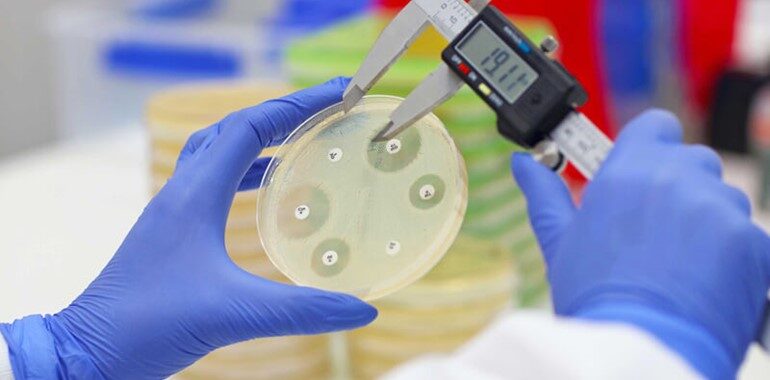
Staphylococcus aureus bacteremia (SAB) is a bloodstream infection caused by Staphylococcus aureus, associated with significant morbidity and mortality. Staphylococcus aureus bacteremia (SAB) continues to be a major cause of community and healthcare-acquired bacteremia. Institutions are striving to improve their standards on infection control and infection prevention of SAB.
Armata Pharmaceuticals, Inc., a biotechnology company focused on the development of high-purity, pathogen-specific phage therapeutics announced positive results from its Phase 1b/2a diSArm trial, which evaluated AP-SA02, a novel intravenous (IV) administered multi-phage therapeutic for the treatment of Staphylococcus aureus bacteremia (SAB), in the “intent-to-treat” population.
The diSArm study (NCT05184764) is a Phase 1b/2a, multicenter, randomized, placebo-controlled, multiple ascending dose escalation study for the safety, tolerability, and efficacy of intravenous AP-SA02 in addition to best available antibiotic therapy (BAT). In this trial, the participants(n=50) were administered AP-SA02 IV every six hours for five days. AP-SA02, given intravenously, was well-tolerated, with no serious adverse events. However, the placebo subjects were considered non-responsive due to either relapse or treatment failure,
The clinical response with AP-SA02 occurred regardless of whether subjects were infected with methicillin-sensitive S. aureus (MSSA) or methicillin-resistant S. aureus (MRSA). All subjects infected with MRSA and treated with AP-SA02 and BAT cleared their infection with no evidence of relapse through the end of the study, when compared to the relapse rate in those treated with BAT alone. A negative blood culture and decline of key predictors of mortality and complications in SAB, including interleukin-10 and C-reactive protein, supported the improvement rate in subjects treated with AP-SA02.
“Firstly, this is the first clear evidence in a randomized controlled trial of the efficacy of phage against a serious systemic pathogen that is responsible for significant morbidity and mortality in the United States, and secondly, Armata was able to successfully produce high titer phage with high purity allowing for repetitive IV administration every six hours without significant safety concerns,” said, Dr. Deborah L. Birx, the Chief Executive Officer and Director at Armata Pharmaceuticals, Inc.
Armata is a clinical-stage biotechnology company focused on developing high-purity pathogen-specific phage therapeutics for the treatment of antibiotic-resistant and difficult-to-treat bacterial infections using its proprietary phage-based technology. Armata is developing and advancing a broad pipeline of natural and synthetic phage candidates, including clinical candidates for Pseudomonas aeruginosa, Staphylococcus aureus, and other pathogens. Armata is working on advancing phage therapy with drug development expertise that spans bench to clinic, including in-house phage-specific current Good Manufacturing Practices manufacturing to support full commercialization.
Check the press release here
For more phage-related global news updates, click here https://www.thephage.xyz/category/news/