
Bacteriophages are viruses that have high specificity for their hosts. Given the fact that their target host is bacteria, it makes them an attractive means of eradicating bacteria infections. These entities are capable of exhibiting a lytic cycle, which means that they kill (lyse) their host cell to disperse the replicated bodies to other hosts and spread infection.
Despite having the most established means of fighting the bacteria i.e. anti-bacterial, bacteriophages are possible “last resort” strategies for treating infections. This has been proved by the ability of these viral particles to treat patients infested by “superbugs”
There are some major limitations to the use of phages for therapy
- The phages are sensitive to changes in pH
- They could be damaged by enzymatic activity caused due to both Bacterial hosts and human immune cells.
- They have a short serum /plasma half-life. (Serum half-life of a drug is the time it takes for the drug’s concentration in blood plasma/serum to reduce to half of the original concentration.)
A novel approach to overcoming the limitations of phage therapy
A possible solution could be seen by loading the phage within a biomaterial (i.e., biopolymers, synthetic polymers, and ceramics). A good example is DendriPrep, A dendrimer innovative technology from New York City State University (NCSU) researchers that improves the delivery of nanoparticles i.e bacteriophages. It can shield the incorporated phage against many harmful environmental factors, as well as provide a controlled release for prolonged serum half-life.
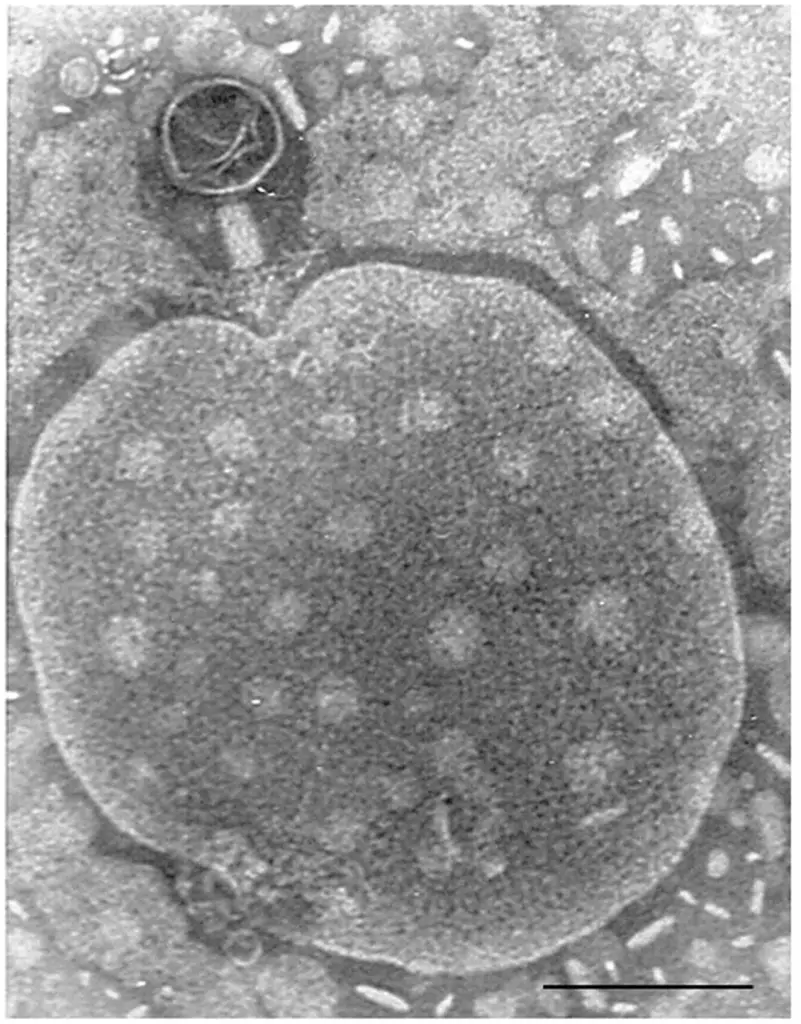
Bacteriophages are specific natural enemies of bacteria although gene manipulation can lead to other purposes in drug delivery and vaccines. Filamentous phages have a huge surface bearing capacity and flexible genetic engineering property, thus, can easily be loaded with drugs and utilized for targeted drug delivery. In nanomedicine, phage-mimetic nanoparticles have as well been developed to achieve the same. In particular, chemical drugs can be conjugated on the phage surface by chemical modification, and gene drugs can also be inserted into the genome of the phage by recombinant DNA technology. This ability of filamentous phages makes them a potential system for cancer therapeutics and treatments.
The huge surface bearing capacity and flexible genetic engineering property in filamentous phages can effectively be used to load chemical and/or generic drugs. These particles can target the specific lesion location in the patient’s body. The peptides and proteins exhibited on the phage surface can moreover be applied directly as self-navigating drug delivery nanovesicles.
Interestingly, the filamentous phage is considered to be an eligible performer in antibody engineering which makes it gain a reputation in nanobiotechnology and cancer research. The property of bacteriophage which makes it an eligible candidate to fight against cancer is its specificity towards certain cells and/ or tissues.
The specificity of the target is related to the surface peptides and proteins on the phage coat, which can further be conjugated with other therapeutic nanoparticles. The genetic and chemical modification of the filamentous phages leads to a modular target drug-carrying platform of nanometric dimensions, where the targeting moieties (biomolecules) and the conjugated drugs can be delivered at will.
Researchers in the study done by Bar et al (2008) used genetically modified and chemically manipulated filamentous phages. The genetic modification endowed the phages with the ability to display a host-specificity-conferring ligand then they were loaded with a large payload of cytotoxic drugs by chemical conjugations. In their experiment, they used anti ErbB2 and anti ERGR antibodies as targeting moieties, the drug hygromycin conjugated to the phages by a covalent amide bond, or the drug doxorubicin conjugated to genetically-engineered cathepsin-B sites on the phage coat. It was observed that targeting phage nanomedicines via specific antibodies to receptors on cancer cell membranes results in endocytosis, intracellular degradation, and drug release, resulting in growth inhibition of the target cells in vitro with a potentiation factor of >1000 over the corresponding free drugs. They used “binding analysis with monoclonal antibodies complexed phage nanoparticles using whole-cell ELISA” as the technique to obtain the results.
In 2019 at the Imperial College of London research has been going on targeting a hopeful cure for Glioblastoma, a form of brain tumour to which currently the only available chemotherapy is a drug called temozolomide (TMZ). However, it has limited effect. An international team led by researchers at Imperial College London has had promising results using modified bacteriophages to target cancer cells in the brains of mice. They were able to deliver targeted therapy directly to cancer and amplify the effect with TMZ. The treatments led to tumours shrinking while leaving healthy tissues intact as well as increasing the life expectancy of the animals.
Case of Oncolytic Viruses
Oncolytic (tumour-killing) viruses a.k.a. OVs induce antitumor effects in a two-way mechanism one by direct lysis of target cells and eliciting an immunogenic response to the virus and ultimately to the target cells. They destroy tumour mass by infecting and multiplying within the mass. In addition to viral lysis, the tumour mass, due to the presence of immunogenic viruses, is subject to attack by the immune system. Partial remission of tumour mass when patients contracted viral diseases was observed by physicians as early as the beginning of the twentieth century. One of the FDA-approved oncolytic viruses is HSV-1 based T-VEC and is clinically in use. Attenuated human pathogens, which have been tested as potential OVs, include the adenovirus, the vaccinia virus, the measles virus, the mumps virus, and the influenza virus. Other viruses known to be poor human pathogens, which have been tested as OVs, include the Newcastle disease virus and the vesicular stomatitis virus. potential OVs are often not powerful enough for solid tumours and safety has not always been matched with efficacy, as OVs are known to exhibit a certain range of toxic effects. In addition, live viruses have been shown to be transferable from the primary treatment patient to healthcare workers and people inhabiting the same household.
Why are phages better?
A major class of OVs are pathogenic to humans and are thus not in use for the purpose. However, studies on bacteriophages have shown the potential of inhibiting tumours. As stated by Budynek et al (2010), In 1940, the accumulation of phages in cancer tissue and the inhibition of tumour growth was observed, and in 1958, the binding of phages to cancer cells was seen both in vitro and in vivo. Phage T4 and its substrain HAP1 were shown to bind melanoma cells and inhibit lung metastasis in a murine model.
Bacteriophages have been proven to be a natural and involuntary medicine as they are circulating in the mammalian body controlling invading pathogens and regulating our immune system naturally. Furthermore, they can block β3 integrin activity on neoplastic cells thus preventing growth and metastasis formation. Moreover, they also restrict angiogenesis in the developing cancerous tissues or organs and prevent metastasis by inhibiting the adhesion of platelets and T-cells to the fibrinogens.
In the study by Hwang et al, T7 bacteriophages were engineered to act as anti-cancer agents. In vivo, their experiment showed significant results in tumour-carrying mice, with half being administered with wild-type T7 phage and half with engineered T7 phage intravenously. About 40% of mice treated with wild-type T7 phage survived while the stats were 100% positive for those treated with engineered T7 phage.
Conclusion
With our increasing understanding of Bacteriophages and their behavior on interaction with the tumor cell lines, we can lead to the development of a minimally invasive method of cancer treatment. Due to the abundant presence of phages in our natural environment phage mediated cancer treatment can also be kept from reaching the gold standards as of those currently available treatments (surgery, chemotherapy, radiotherapy, etc.). It can be hoped that the development of bacteriophage-mediated cancer therapy can provide humanity with the potential of cancer prevention affordably, as everyone owns an equal right to live.

Can that technology be applied to every disease or it's for cancer only?
Hi, Stanford. thank you for your query. The approach mentioned in the article is directed towards cancer, and it is to be noted that not all kinds of cancers are yet subjected to such a treatment as research is still ongoing. But bacteriophages are viruses that particularly target bacteria, and in major cases kill the bacteria. There have been some cases where phages have been used to treat bacterial infections, after genetic and chemical modifications.
bacteriophages are considered to be potential target based drug carriers in many fields.
I hope this helps. 🙂
It's so exciting. We need more innovation in Cancer treatment
[…] Phage mediated therapy for cancer This article talk about how phages can be utilized to treat cancer […]
[…] Phage mediated therapy for cancer […]